New European Study: Restoring NAD+ with NMN Rescues Immune Cell Function Against Hepatitis B
Treating immune cells that target hepatitis B with nicotinamide mononucleotide (NMN) restores their DNA repair mechanisms and antiviral function.
Highlights
- The nicotinamide adenine dinucleotide (NAD+) precursor NMN improves hepatitis B virus-specific immune cell (CD8 T cell) antiviral activation.
- Exhausted hepatitis B-specific CD8 T cells with impaired function exhibit high levels of DNA damage and defective DNA repair mechanisms.
- In their exhausted state, CD8 T immune cells display higher NAD+-consuming CD38 enzyme levels and lower antiviral protein production.
Hepatitis B typically resolves within six months for most people infected, but chronic hepatitis B that doesn’t go away can occur due to CD8 T cell exhaustion. CD8 T cell exhaustion results from high levels of exposure to a pathogen like the hepatitis B virus, triggering T cell DNA damage and a dysfunctional antiviral response. Identifying the cellular processes behind CD8 T cell exhaustion can help with developing therapies for chronic hepatitis B. Some researchers have recently proposed that NAD+ depletion underlies T cell exhaustion.
Published in the Journal of Hepatology, Fisicaro and colleagues from the University of Parma in Italy demonstrate that treating CD8 T cells specific for the hepatitis B virus with NMN improves their production of antiviral proteins – cytokines. Exhausted hepatitis B-specific CD8 T cells were found to have high levels of DNA damage along with dysfunctional DNA repair mechanisms. Findings from the study suggest that NMN and/or CD38 inhibitors can be used to revamp exhausted T cells to ward off chronic hepatitis B, along with potentially other viral infections.
NMN Restores Immune Cell Antiviral Activation
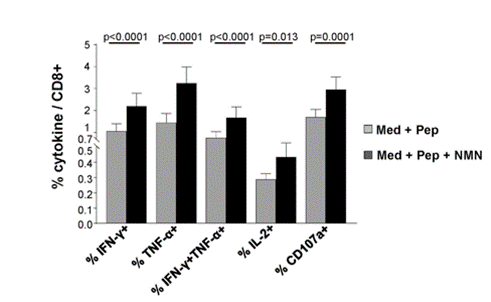
Because Fisicaro and colleagues hypothesized that restoring NAD+ levels with NMN could revamp exhausted CD8 T cells, they isolated these immune cells from chronic hepatitis B patients for testing. Upon treatment with NMN, the cells expressed more antiviral cytokines, specifically a 2.7-fold increase for cytokine interferon gamma (IFN-𝛾), suggesting that NMN restores the CD8 T cells’ antiviral properties.
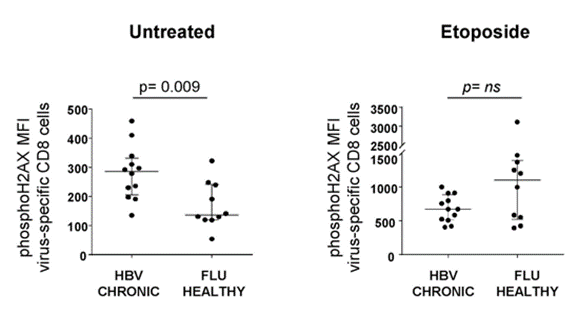
To examine the cellular damage that goes along with T cell exhaustion in chronic hepatitis B infection, Fisicaro and colleagues compared DNA damage in hepatitis B-specific T cells with influenza (FLU)-specific T cells. The researchers found significantly more DNA damage in the hepatitis B-specific T cells. They also found that treating the cells with a DNA damage-inducing molecule (etoposide) elicited a trend toward a lower DNA damage response compared to FLU-specific T cells. Since DNA damage responses like the one mediated by PARPs require NAD+, this data suggests that NAD+ deficiency is tied to higher levels of DNA damage in exhausted hepatitis B-specific CD8 T cells.
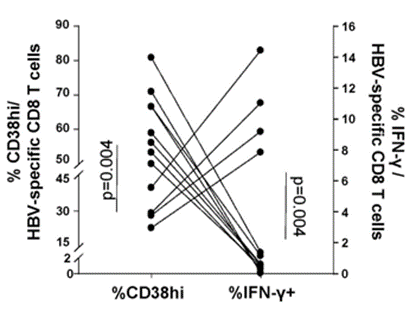
To explore further how NAD+ repletion with NMN improves exhausted CD8 T cell antiviral cytokine production, Fisicaro and colleagues measured how high levels of the NAD+-consuming CD38 enzyme relate to cytokine levels. They measured one of the primary cytokines – IFN-𝛾 – and found that high CD38 enzyme levels are associated with low IFN-𝛾 levels. These data provide evidence that high CD38 enzyme levels can lead to lower antiviral cytokine production. This also lends credulity to the assertion that low NAD+ levels play an important role in chronic hepatitis B pathology since high CD38 levels would theoretically lower cellular NAD+.
“Our data show increased DNA damage with limited activation of the DNA repair machinery in [hepatitis B virus]-specific CD8 T cells from [chronic hepatitis B] patients,” say Fisicaro and colleagues. “This strongly suggests that NAD-consuming enzymes, particularly overexpressed CD38, may play a pivotal role in NAD depletion. Reconstitution of many interconnected intracellular functions by NMN supplementation indicates that NAD depletion likely represents an important determinant of T cell exhaustion.”
NMN May Revamp Immune Cells in Multiple Types of Infections
The study provides evidence that the hepatitis B virus triggers higher CD38 enzyme activation, leading to lower NAD+ levels. Since DNA repair mechanisms like PARPs require NAD+ for their function, this could help explain why exhausted hepatitis B virus-specific T cells have more DNA damage. This could also help explain why DNA repair mechanisms don’t work as well in exhausted hepatitis B-specific CD8 T cells.
Treating CD8 T immune cells with the NAD+ precursor NMN restores antiviral cytokine production, suggesting that NAD+ repletion reconstitutes their function. NMN’s capability to revamp immune cell function provides support that NAD+ depletion contributes to CD8 T cell exhaustion and dysfunction. Whether NMN provides beneficial effects in exhausted CD8 T cells in other types of infections should be explored. If NMN can restore exhausted T cells in other infections, it may be the case that NAD+ makes it easier for immune cells to fight other types of infection, also.
Model: Hepatitis B-specific CD8 T cells from chronic hepatitis B patients

