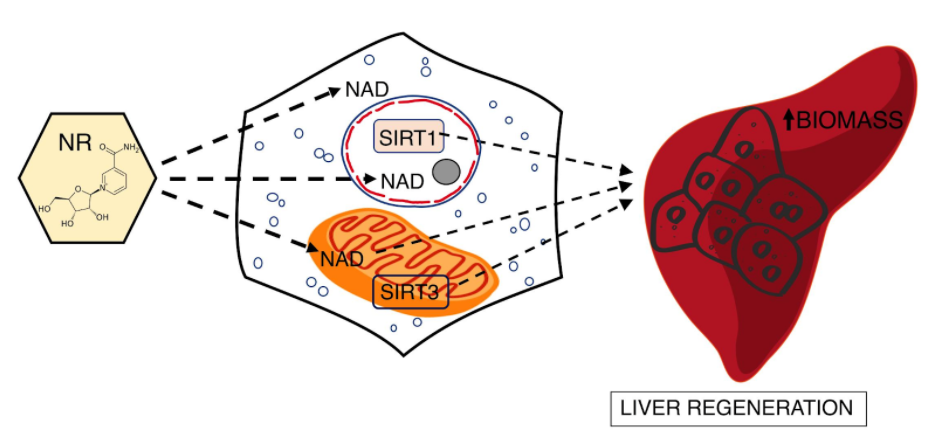
NAD+ Supplementation Improves Liver Regeneration
Sirtuin proteins are not required for the NAD+ precursor nicotinamide riboside to enhance liver regeneration
Highlights
· Loss of the mitochondrial sirtuin SIRT3 impairs liver regeneration after partial liver removal.
· The NAD+ precursor nicotinamide riboside can regenerate the liver independent of SIRT3.
Our livers are constantly fighting off damage. To win this war and keep our body metabolism running properly, liver cells lost due to acute injury or over the course of chronic disease are replaced by replicating mature cells. The ability to activate the acute regenerative response can be the determining factor between survival and death after a toxic or traumatic injury, transplant, or surgical resection. Despite decades of study, there remains no clinically proven therapy to support regeneration in patients at risk for liver failure or following an acute injury or surgical intervention.
Mukherjee and colleagues from the University of Pennsylvania published an article in JCI Insight showing that liver cell loss of longevity-associated enzymes called sirtuins drastically impairs regeneration after partial removal of the liver. Despite the dependence of these enzymes on the vital bioenergetic molecule nicotinamide adenine dinucleotide (NAD+) and critical roles in regeneration, SIRT1 and SIRT3 are not required for NAD+ precursors to enhance liver regeneration. Along these lines, the NAD+ precursor nicotinamide riboside (NR) rapidly increases liver cell function.
“We provide the first evidence for an essential role for a mitochondrial sirtuin during liver regeneration and insight into the beneficial effects of NR,” proposed Mukherjee and colleagues. “We identify SIRT3 as a critical new player in liver regeneration and show that NAD+ supplementation influences metabolism independently of both SIRT1 and SIRT3.”
What do we know about the role of NAD+ and sirtuins in liver regeneration?
NAD+ serves as a co-substrate for several classes of enzymes with important signaling functions. These include the sirtuins (SIRT1-7), a family of enzymes dependent on NAD+ that differ in their tissue distribution, subcellular localization, and enzymatic activity.
Within liver cells, the switch from a non-dividing to a dividing state involves substantial remodeling of metabolism. Plus, the birth of new liver cells must be accomplished while the remaining tissue supports multiple indispensable liver functions. Although liver regeneration is an energetically demanding process that requires substantial flux through mitochondrial pathways, the role of mitochondrial sirtuins has not been investigated.
SIRT3 has emerged as the major sirtuin responsible for modifying mitochondrial proteins and loss of SIRT3 renders mice susceptible to ischemic and other injuries. SIRT3 has been shown to influence the metabolic capacity of mitochondria under stresses, including in liver cells, and is responsible for the ability of NR to protect against hearing loss in mice suggesting that it might also mediate the effect of NR on liver regeneration.
The beneficial effects of NR in the regenerating liver are independent of SIRT1 and SIRT3
Mukherjee and colleagues looked at how sirtuins localized to different cellular compartments participated in liver regeneration in the context of NAD+ supplementation. The majority of cases where a mechanism has been suggested for beneficial effects following NAD+ supplementation involves SIRT1 or in rarer cases SIRT3. SIRT1 has previously been implicated in liver regeneration and in resolving lipid accumulation in liver cells, which promotes metabolic dysfunction throughout the body, following NR treatment in the setting of non-alcoholic fatty liver disease.
For these reasons, Mukherjee and colleagues initially set out to test whether the beneficial effects of NR in liver regeneration are dependent on SIRT1. As expected, they confirmed that the loss of SIRT1 activity in liver cells substantially impaired liver regeneration and delayed lipid clearance. However, NR treatment improved liver weight, liver cell replication, and lipid clearance even in the livers lacking SIRT1. Thus, NR appears to improve the replicative capacity of liver cells in a manner that is independent of and additive with liver SIRT1 activity.
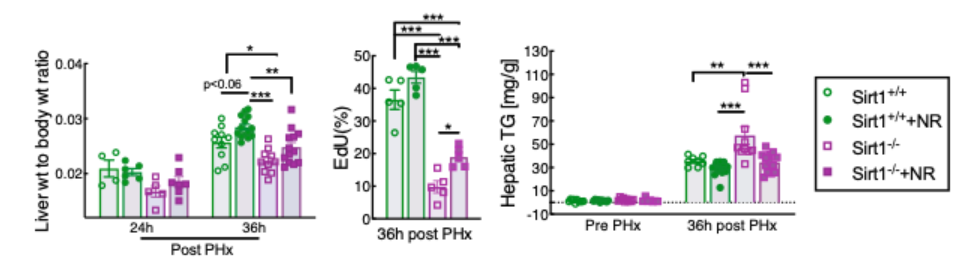
The effect of SIRT3 on liver regeneration has not previously been investigated in animal models, let alone humans. So, Mukherjee and colleagues generated mice with liver cells lacking SIRT3, which drastically impaired liver cell replication and promoted fat accumulation. These findings reveal an important and unrecognized role for SIRT3 in liver regeneration. But, just like with SIRT1, NR treatment remained fully effective in these mice with liver cells lacking SIRT3 for all of the parameters assayed, indicating an independent mechanism of action.
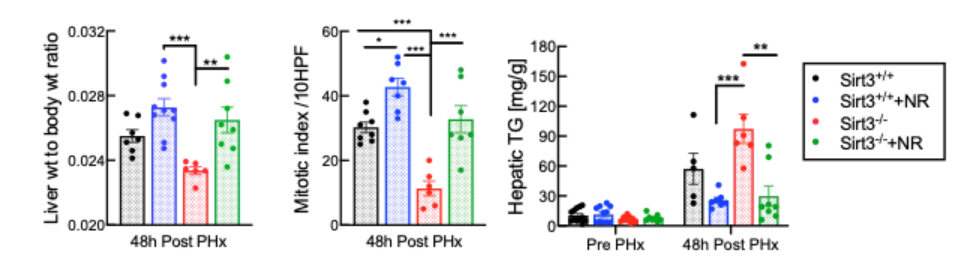
This study shows that the loss of SIRT3 results in mitochondrial dysfunction, lipid accumulation, and loss of liver cell replication in the regenerating liver. While increasing NAD+ concentration with precursors like NR is sufficient to accelerate mitochondrial metabolism in liver cells or the regenerating liver, the effect is independent of both SIRT3 and its nuclear counterpart SIRT1.
Although it remains formally possible that another enzyme that consumes NAD+ contributes to the effects of NR in mice, these data are consistent with the view that the direct role of NAD+ in mitochondrial reactions is limiting in liver regeneration. More generally, the ability of NAD+ concentration to influence flux through mitochondrial pathways in this system supports the model that metabolic reactions may be directly influenced by physiologically relevant changes in NAD+ concentration. These findings support the role of NAD+ as a node to many biological processes critical to the viability of our cells and, thus, ourselves.