NAD+ Precursors May Counteract Vision Loss in Glaucoma
An Italian researcher describes an inherent structural vulnerability within the eye, lowering NAD+ and contributing to an eye disease, which can be rectified with NAD+ precursors.
Highlights
- Preclinical trials in mice suggest glaucoma, an eye disease characterized by damage to nerves signaling visual information to the brain, begins with the deterioration of neuronal projections (axons) from eye cells (retinal ganglion cells [RGCs]) that relay visual information to the brain.
- RGC axonal deterioration is a result of low nicotinamide (NAM) adenine dinucleotide (NAD+) from restricted nutrient trafficking within these axons.
- Early clinical trials in humans suggest supplementing with NAD+ precursors like NAM can help RGCs and could lead to improvements in vision when paired with other, more well established treatments.
Glaucoma prevalence is expected to increase within the next decade, affecting nearly 11 million patients worldwide. Sadly, 9% to 12% of glaucoma patients develop blindness. Surgical interventions to relieve elevated intraocular pressure (pressure in the eyes) have not been successful at alleviating glaucoma-related visual impairment and blindness, so finding more effective treatment options is paramount.
Published in Trends in Pharmacological Sciences, Alberto Chiarugi, professor of Clinical Pharmacology and Oncology at the University of Florence, evaluates the current research and claims glaucoma begins with the deterioration of RGC axons located near the retina, the light-sensitive layer of eye cells located toward the back of the eye. RGC axons are projections from a layer of RGC cells on the back of the eye that allow for vision. He proposes that the lamina cribrosa, an essential part of the eye for maintaining structural integrity, impairs the eyes’ circulation. This scenario leads to decreased delivery of nutrients as well as decreased trafficking of NAD+ synthesis enzymes from the neuronal cell bodies to RGC axons, leading to lowered axonal NAD+ with age. To remedy impaired NAD+ synthesis and preserve RGC axons from deterioration, Chiarugi reviews human studies suggesting that NAD+-boosting supplements like NAM slow and possibly reverse vision loss from glaucoma. This new view of glaucoma disease progression and the potential of NAD+ precursors to reverse it may offer a new glaucoma treatment.
A Small Opening at the Back of the Eye Restricts Circulation to RGC Axons
To unveil his conception of glaucoma disease progression, Chiarugi presents evidence from mouse studies pointing to the deterioration of RGC axons as a key contributor to glaucoma. This goes against the prevailing theory that increased intraocular pressure is the main contributor of vision decline during glaucoma.
To understand why RGC degeneration may lead to glaucoma, Chiarugi examined the structure of the eye through which axons project from RGCs. This structure — the lamina cribrosa — is a narrow opening at the back of the eye that allows axonal projections to relay visual information from the eye to the brain. The structure represents what Chiarugi refers to as a locus minoris resistentie, another way of saying this region is more vulnerable to damage than other parts of the body. Through the evolution of this opening at the back of the eye, structure was traded for function. Structurally, the small diameter of the lamina cribrosa prevents smaller nerves around the optic nerve from dying, forming a cup of dead neurons, in a process called optic nerve cupping. However, functionally, it is so narrow that it slows and can even restrict the flow of nutrients to the RGC axons.
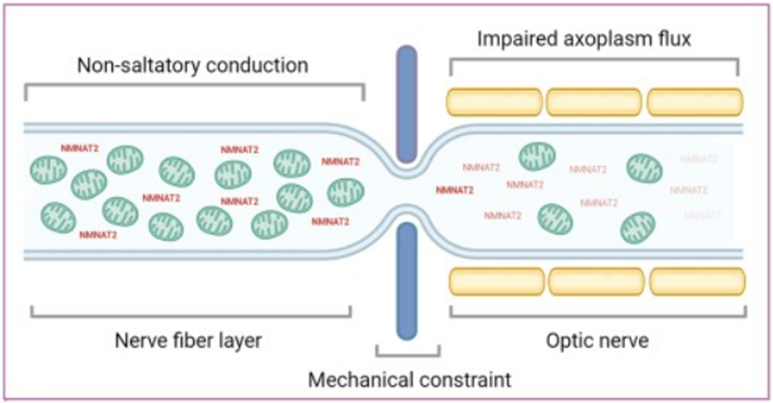
The constrictive nature of the lamina cribrosa bends RGC axons and impedes their uptake of nutrients, mitochondria, and NAD+ synthesis enzymes — NMNAT2 — from neuronal cell bodies. This scenario facilitates lower NAD+ levels along with declining cellular energy within axons.
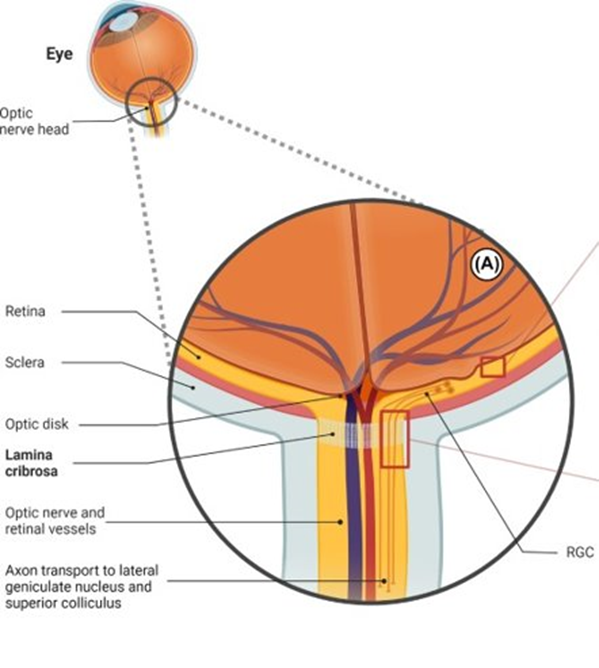
RGC axons don’t have the fatty substance called myelin wrapped around them which speeds the propagation of their signaling. For this reason, they require more energy for their signaling function as opposed to myelinated axons that require less energy for signaling. Along those lines, they require copious NAD+, which is used for cellular energy generation, to produce energy for the optic nerve. With the lamina cribrosa pinching and restricting the transport of nutrients, mitochondria, and the essential NAD+ synthesis enzyme — NMNAT2 — for energy generation, this scenario precipitates a lack of NAD+ and RGC axon energy and ultimately drives axonal degeneration.
Human Trials Suggest NAD+ Precursors Benefit Glaucoma Patient Vision
Interestingly, a human study has shown that supplementing with NAD+ precursors like NAM to boost NAD+ in RGC axons may mildly improve vision in glaucoma patients. This study showed that NAM and pyruvate — an important molecule for cellular energy generation — supplementation improved vision in glaucoma patients. In this study, NAM and pyruvate were administered at 1.0 to 3.0 grams per day and 1.5 to 3.0 grams per day, respectively, for three weeks. Overall, the study’s results showed that NAM and pyruvate supplementation improved vision.
What’s more, another three-year nutritional study utilizing 5,780 individuals reported that increasing dietary niacin, another NAD+ precursor, to approximately 28 mg per day significantly reduced the odds of glaucoma onset. These data suggest that supplementing with NAD+ precursors is associated with a decreased likelihood of developing glaucoma.
Furthermore, these positive results have propelled ongoing human trials exploring whether supplementing with another NAD+ precursor, NR, improves the ability to detect light in different regions of the visual field in glaucoma patients. This study includes NR supplementation at 300 mg per day over a 24-month period. Furthermore, three additional ongoing clinical trials are examining whether supplementing with two grams per day of oral NAM alone or with pyruvate slows glaucoma progression (NCT05275738, NCT05405868, and NCT05695027). Such clinical trials will help solidify whether NAD+ precursor supplementation with either NAM or NR can slow glaucoma progression and even help restore the visual field.
Using NAD+ Precursors to Preserve Eye Function
The Italian researcher Alberto Chiarugi has shed light on a potential new view of glaucoma where the primary contributor to the disease is not high fluid pressure within the eye but rather RGC axonal deterioration from a lack of NAD+. The lamina cribrosa, especially under high pressure from eye fluid buildup, can impair the delivery of nutrients and NMNAT2 enzymes with essential roles in NAD+ synthesis from neuronal cell bodies to RGC axons. Such a scenario impedes energy generation in this axonal region with a high energy demand pertaining to relaying visual signals to the brain. Since RGC axons already lack myelination for high-speed signal propagation, losing NAD+-associated cell energy production with age leads to their degeneration.
Intriguingly, human trials have already provided some hope that the NAD+ precursor NAM can improve vision in patients with glaucoma. Ongoing clinical trials may provide further evidence that increasing NAD+ levels can serve as a means of slowing glaucoma progression and preserving or even improving vision.

