Study Shows that NAD+ Precursors Reverse Blood Vessel Dysfunction
Nicotinamide mononucleotide represents an effective protective mechanism to maintain healthy endothelial cells
Some nicotinamide adenine dinucleotide (NAD+) precursors have the potential to be vasoprotective — they can act to alleviate or prevent conditions or diseases that affect the blood vessels. Despite many studies on the benefits of two of these NAD+ precursors nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), the effects on the endothelium — the cells that line blood vessels — are not completely understood.
Mateuszuka and colleagues from Jagiellonian University in Krakow, Poland, published an article in the journal Biochemical Pharmacology characterizing the effects of NMN and NR on endothelial inflammation and dysfunction. Their research demonstrated that both NMN and NR modulated intracellular NAD+ content in the endothelium and inhibited endothelial inflammation. NMN’s effects on the endothelium were mediated by conversion of NMN to NR via the cell surface receptor CD73. Importantly, this work shows that these NAD+ precursors are an effective vasoprotective mechanism to maintain healthy endothelial cells.
A tale of two NAD+ precursors
The NAD+ precursors NR and NMN have drawn attention for their numerous beneficial effects in various settings. However, their bioavailability and pathways of metabolism towards NAD+ differ.
NR is detectable in cow milk, milk-derived products, and in natural products containing yeast. It has a good bio-availability and intracellularly is metabolized to NMN.
In contrast to NR, NMN has a worse bioavailability because it is unable to pass through the endothelial membrane. NMN has to be modified outside of the cell — extracellularly — to NR by a cell surface receptor called CD73 or metabolized to nicotinamide (NA) by extracellular CD38 prior to crossing into endothelial cells.
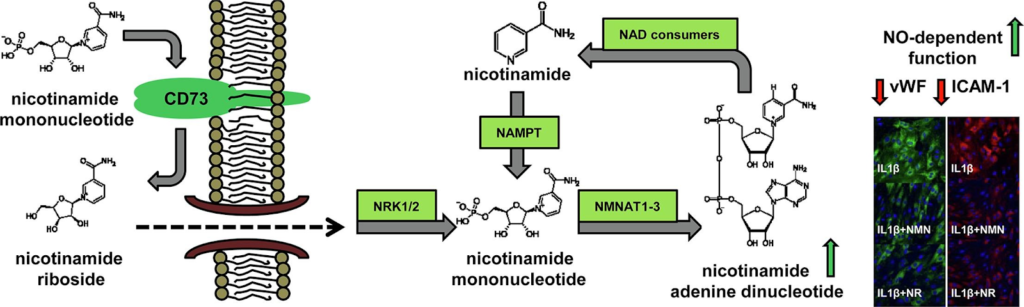
But the role of the enzymes CD73 and CD38 in NMN-induced effects has not been fully characterized. So, it is not clear whether NMN-triggered beneficial effects are NR- or NA-dependent and what metabolic enzymes are involved.
In the present work, Mateuszuka and colleagues aimed to characterize how NMN and NR affect endothelial cells, including endothelial inflammation and dysfunction. In addition, they paid particular attention to how NMN was converted to exert its effects.
NMN conversion to NR by CD73 protects endothelium from inflammation and dysfunction
The Polish research team demonstrated the anti-inflammatory effects of NMN, that was comparable to the effects of NR in cultured endothelial cells. They then demonstrated that endothelial inflammation was associated with the upregulation of CD73 and NAMPT, the enzyme that converts NA to NMN inside of cells. This suggests that intracellular NMN synthesis by NAMPT from nicotinamide and extracellular conversion of NMN to NR represent the major NAD+ synthesis systems activated in endothelial inflammation.
Furthermore, Mateuszuka and colleagues showed that NMN-afforded effects but not NR-induced effects were absent when CD73 was inhibited or in CD73-deficient mice. They demonstrated that the CD73 inhibitor AOPCP effectively diminished NR production from NMN in two types of endothelial cells, but had almost no effect on NA production from NMN.
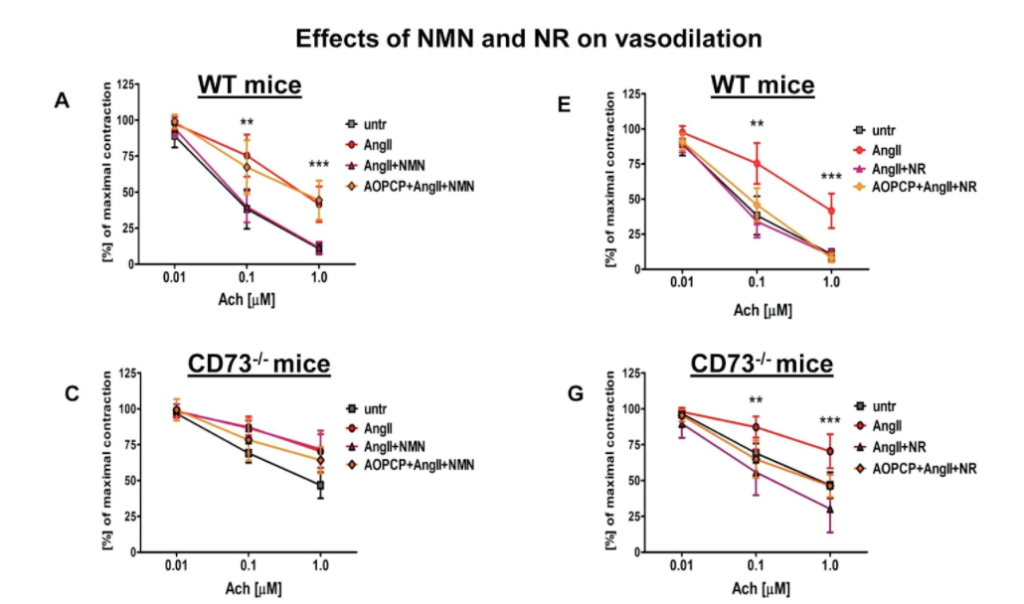
The research team then examined aortic rings that were taken from mice and cultured in the lab with the vasoconstrictor angiotensin II for 24 hours. They observed that the vasorelaxation induced by acetylcholine was impaired. But NMN and NR both prevented angiotensin II-induced endothelial dysfunction in the aorta. Also, in aortic rings taken from CD73 knockout mice, the NMN effect was lost whereas the NR effect was preserved.
Vascular pathologies where CD73 is altered needs to be elucidated in further studies
In conclusion, the researchers demonstrated that the anti-inflammatory and vasoprotective effects of NMN were comparable to the effects of NR in cultured endothelial cells as well as in the aortic rings taken from mice. “We demonstrated the nicotinamide mononucleotide reversed endothelial dysfunction and inflammation by extracellular conversion to nicotinamide riboside via CD73, whereas nicotinamide riboside-induced effects were CD73-independent,” said the authors in the study.
“Altogether, our results point to the extracellular conversion of NMN to NR by CD73 localized in the luminal surface of endothelial cells as important vasoprotective mechanisms to maintain intracellular NAD+,” concluded the authors in the study. Further studies are needed to determine the relative importance of CD73-dependent regulation of NAD+ metabolism in mice and humans. The importance of mechanisms dependent on NAD+ in vascular disorders where CD73 is altered needs to be elucidated in further studies.

