Increased NAD+ Promotes Sensitivity to Anti-Cancer Therapy
NAD+ supplement combined with PD-L1 antibody provides a novel therapeutic strategy for immunotherapy-resistant tumors
All cells need nicotinamide adenine dinucleotide (NAD), a bioenergetic molecule, to carry out essential functions and for survival. But NAD+ production (biosynthesis) is often elevated in human cancers, where it plays a critical role in the initiation, progression, and relapse of tumors. Yet, how NAD+ metabolism — the chemical reactions that make, process, and use NAD+ — plays a role in the regulation of tumor progression through its evasion of the body’s immune response has remained largely unknown.
Wang and colleagues from the Secondary Military Medical University in Shanghai, China, published a study in Cell Metabolism where they provided evidence for a cellular mechanism whereby NAD+ synthesis in cells promotes tumor progression. Their experiments showed that supplementation with the NAD+ precursor nicotinamide mononucleotide (NMN) increased NAD+ levels and improved the effectiveness of a therapeutic option for tumors resistant to immunotherapy, a cancer treatment that helps the immune system fight off tumors.
In their study, the researchers provided experimental evidence from mice that decreased levels of a key enzyme in the NAD+ biosynthesis pathway, NAMPT, led to enhanced activation of white blood cells that attack tumors called CD8+ T cells. The activation of these T cells facilitated their function to combat tumors. The reduction in the NAMPT enzyme levels was accompanied by reductions in cellular levels of NAD+, indicating that NAD+ metabolism controls the CD8+ T cell function against tumors.
Their data also showed that NAD+ metabolism can drive tumor evasion from the immune system by promoting levels of a protein on cancer cells called PD-L1. The presence of PD-L1 on the surfaces of cancer cells inhibits the function of T cells by binding to receptors on the T cell surface called PD-1. The interaction between PD-L1 on cancer cells and PD-1 on T cells leads to T cell exhaustion, which is when T cells lose their ability to kill cancerous cells from binding too many foreign particles from tumors, and evasion of the immune response by cancer cells. The team then found that more NAMPT in tumor cells correlated with higher levels of the PD-L1 protein found on the surfaces of cancer cells and a higher incidence of tumor evasion from the immune response.
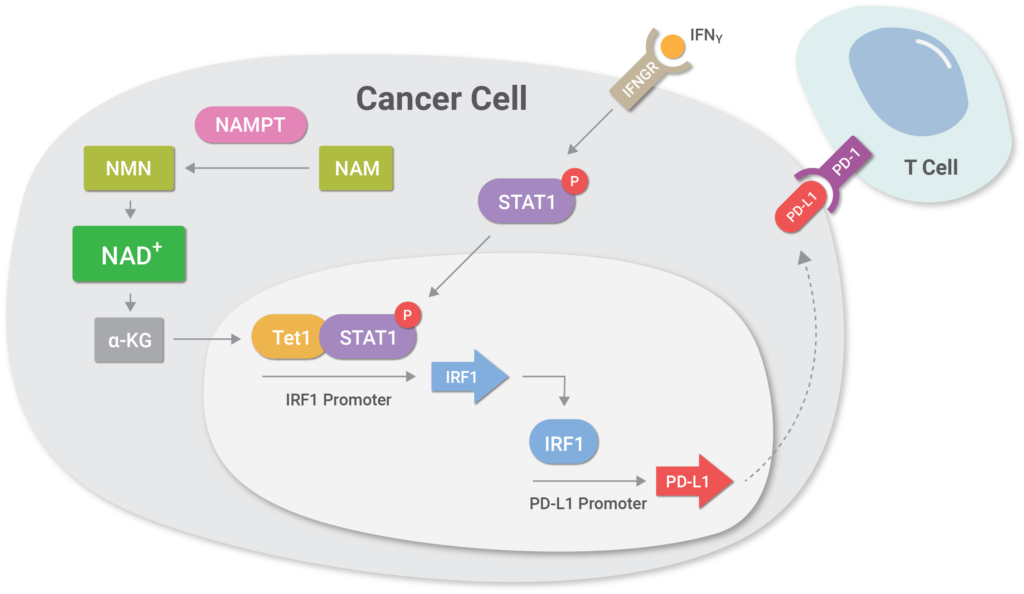
(Lv et al., 2020 | Cell Metabolism) NAD+ metabolism controls the evasion of tumor cells from the immune response. Higher levels of the enzyme NAMPT promotes NAD+ biosynthesis, which triggers a cellular cascade called the NAMPT-TET1-p-STAT1-IRF1-PD-L1 axis that promotes higher levels of the protein PD-L1 on cancer cell surfaces. Essentially, the cellular cascade facilitates the binding of a protein called IRF1 to a region of DNA that codes for PD-L1, inducing higher levels of PD-L1 protein levels on the surface of tumor cells. PD-L1 binds to the PD-1 receptor on T cells, leading to T cell exhaustion with loss of T cell function and subsequent tumor cell evasion from the immune response.
NMN Supplementation Enhances Anti-Tumor Immunity
What’s more, the researchers’ results suggest that replenishing cellular NAD+ levels with NMN supplementation can enhance the effects of immune system-enhancing therapies meant to inhibit PD-L1 function. This is because higher NAMPT expression levels could predict better efficacy of these therapies, most likely due to NAMPT’s role in NAD+ synthesis in cells which promotes the presentation of PD-L1 on tumor cell surfaces. When the group supplemented cancerous cells from mice in Petri dishes with NMN, they found that this method of NAD+ replenishment sensitized the tumors to immunotherapy. The scientists treated the tumors supplemented with NMN with protein components of the immune system called antibodies against PD-L1 as an anti-cancer therapy, which significantly inhibited tumor progression.
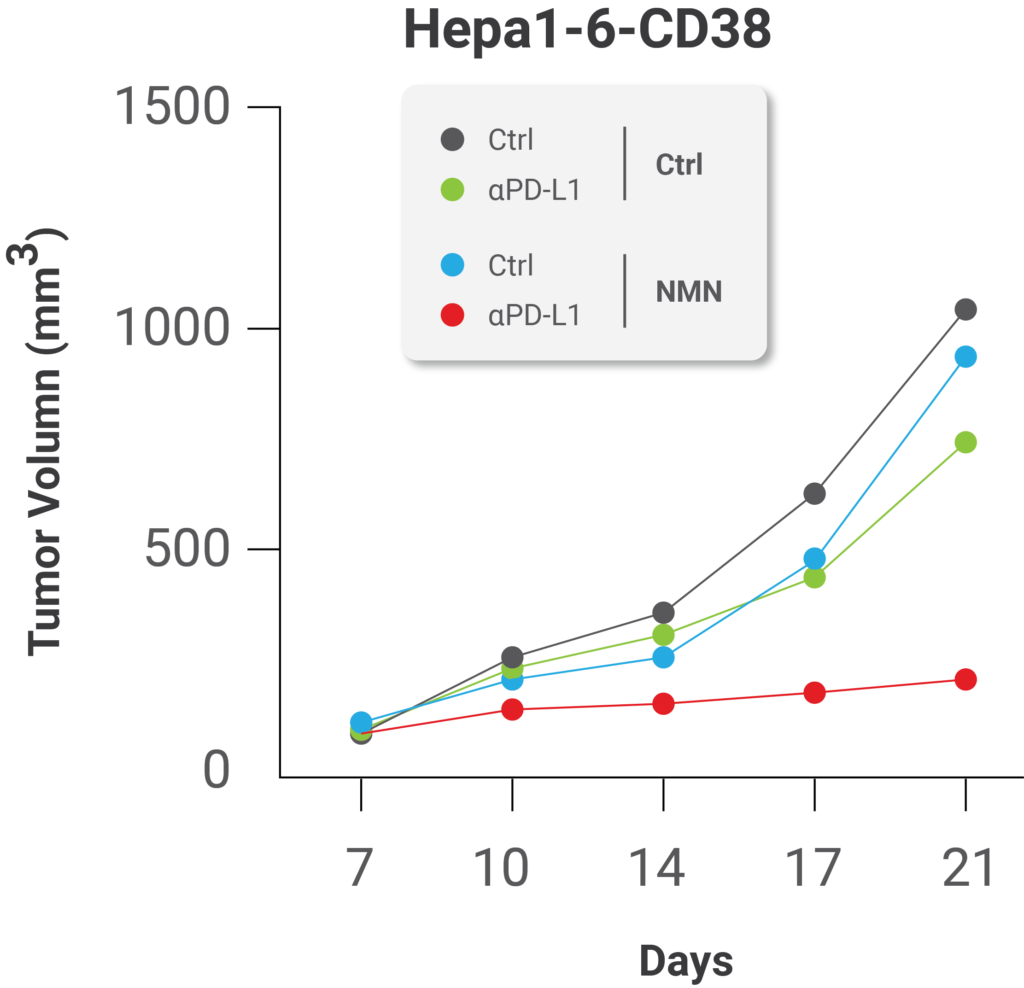
(Lv et al., 2020 | Cell Metabolism) NMN treatment enhances the effect of immunotherapy against cancer. NMN treatment significantly enhanced the anti-cancer effect of PD-L1 antibodies, protein components of the immune system.
This study clarifies the role of NAD+ metabolism in regulating tumor immune evasion, with elevated levels of NAD+ promoting the presentation of the protein PD-L1 on tumor cells and subsequent immune cell exhaustion. What’s more, the results indicated that increasing NAD+ levels with its precursor NMN can enhance tumor sensitivity to cancer immunotherapy.
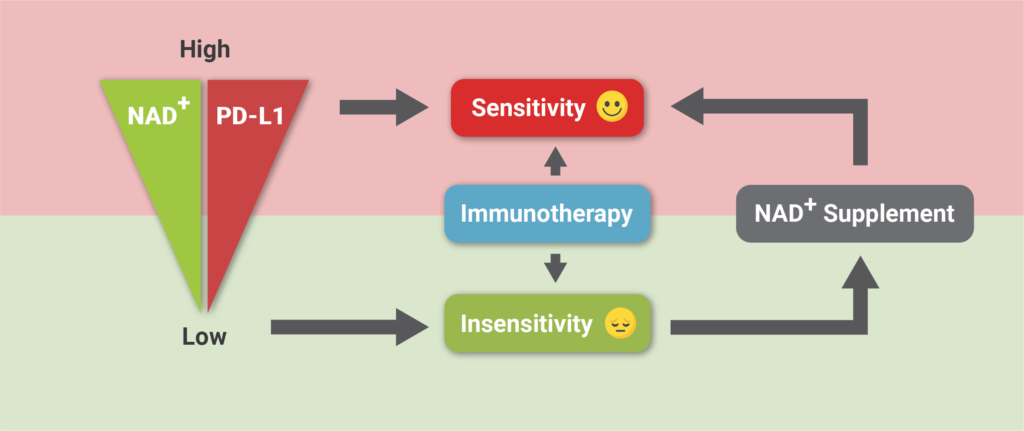
(Lv et al., 2020 | Cell Metabolism) Increasing NAD+ levels with NMN supplementation improves tumor sensitivity to anti-cancer therapy. Lower levels of NAD+ result in lower levels of the PD-L1 protein presented on tumor cells and insensitivity to immunotherapy. Supplementation with NAD+ increases NAD+ and PD-L1 protein presence on cells, which enhances sensitivity to anti-cancer immunotherapy.
“NAD+ supplement combined with PD-L1 antibody provides a novel therapeutic strategy for immunotherapy-resistant tumors,” stated Wang and colleagues in their report.
Still, they performed this research in animal tumor cells, which can only partially recapitulate the pathophysiology of human tumors. Whether NAD+ supplementation combined with PD-L1 works as a therapeutic strategy will need to be verified in human clinical trials.

