NAD+ Improves Adult Stem Cells For Research and Clinical Applications
Study shows that NAD+ supplementation postpones the senescence of bone marrow-derived stem cells
Highlights
- Nicotinamide adenine dinucleotide (NAD+) replenishment significantly postpones the senescence of bone marrow-derived mesenchymal stem cells (BMSCs).
- NAD+ supplementation reduced cell levels of harmful oxygen-containing molecules called reactive oxygen species (ROS) in senescent BMSCs.
Stem cells are found throughout our adult bodies that multiply to replenish dying cells and regenerate damaged tissues. One type known as bone marrow-derived mesenchymal stem cells (BMSCs) can give rise to cells that make up all sorts of tissues, including bone, tendon, cartilage, fat, muscle, and blood vessels, to name a few. BMSCs have great therapeutic potential for tissue engineering and stem cell transplantation. But, there are few BMSCs in the body, and those cultured in labs for expansion are prone to an arrest in cell replication, what scientists call senescence, limiting their application in research and the clinic. So, it is particularly important for us to figure out how to delay the aging of BMSCs to make the most of their therapeutic potential.
Here, Wang and colleagues published an article in Antioxidants where they find that nicotinamide adenine dinucleotide (NAD+) replenishment significantly postpones BMSC senescence. NAD+ supplementation reduced cell levels of harmful oxygen-containing molecules called reactive oxygen species (ROS) in senescent BMSCs. Further investigation showed that supplemental NAD+ weakened BMSC senescence by increasing levels of the anti-senescent, longevity-linked enzyme sirtuin 1 (Sirt1), which, when blocked, abolished the beneficial effects of NAD+ in postponing BMSCs senescence.
“Taken together, our results indicate that exogenous NAD+ could postpone … BMSC senescence through Sirt1 signaling, providing a potential method for obtaining high-quality BMSCs to support their research and clinical application.”
NAD+ and Cell Senescence
The current methods of delaying cell senescence mainly focus on interfering with aging-related genes and protein levels or caloric restriction by adding drugs. NAD+ is a common coenzyme of multiple metabolic enzymes in all living cells. It is involved in regulating various body- and disease-related activities, such as cell material metabolism, energy synthesis, and DNA damage repair.
Meanwhile, NAD+ is also a substrate of Sirt1, a key mediator for postponing cell senescence and promoting cell longevity. Studies have found that NAD+ shows a downward trend during cell senescence and that adding NAD+ and its precursors, such as nicotinamide mononucleotide (NMN), has great potential for the treatment of aging-related diseases caused by a decline in NAD+. Supplementation with NAD+ can effectively increase the levels of Sirt1 and protect cells from damage induced by oxidative stress. This suggests that NAD+ replacement therapy may be suitable for targeting aging-related metabolism dysfunction.
Although previous studies have shown that NAD+ plays an important role in various types of cellular senescence, there have been no reports on the effects of exogenous NAD+ on BMSC senescence. That’s why in this study Wang and colleagues focused on exploring whether and how NAD+ supplementation protects BMSCs from senescence.
Supplemental NAD+ Delays BMSC Senescence
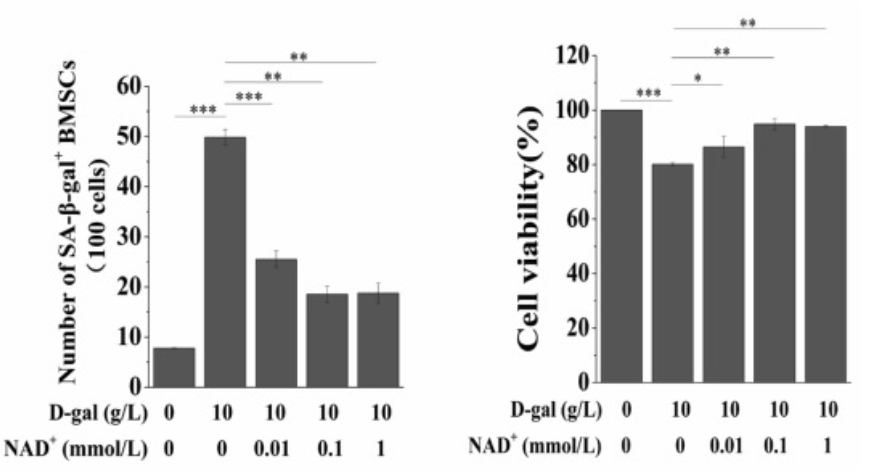
To investigate the effect of supplemental NAD+ on senescent BMSCs, the research team from Chongqing University in China treated the cells with D-galactose, which induces senescence by disrupting mitochondrial function, and different concentrations of NAD+ (0.01, 0.1, and 1 mmol/L). When they looked at the activity of senescent markers and cell viability, the researchers saw major reductions in the number of senescent cells and upswings in cell viability as the NAD+ concentration increased. Besides, the different concentrations of NAD+ had no obvious toxic effects or side effects on normal BMSCs. These results suggest that supplemental NAD+ increases the cell viability of BMSCs and protects them from senescence.
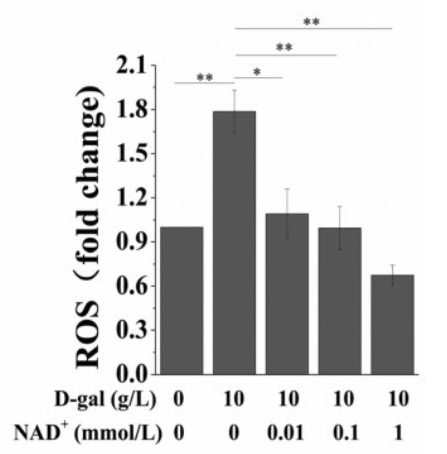
Oxidative stress is an important cause of cell senescence. High concentrations of ROS cause oxidative damage to various organelles and induce cell senescence or death. It is known that D-galactose generates a large amount of ROS, which causes cell dysfunction and promotes cell senescence. Therefore, Wang and colleagues tested the effect of supplemental NAD+ on intracellular ROS in senescent BMSCs. The results showed that D-galactose significantly upregulated cell levels of ROS, while exogenous NAD+ reversed this phenomenon.
Sirt1 Mediates Some Effects of NAD+ on BMSC Senescence
To determine whether Sirt1 was involved in the protection of senescent BMSCs by supplementing NAD+, Wang and colleagues first examined the levels of the Sirt1 protein in senescent BMSCs with or without supplemental NAD+. In senescent BMSCs, Sirt1 levels were significantly downregulated, but supplemental NAD+ restored Sirt1 gene activity and protein levels.
Wang and colleagues wanted to also understand if Sirt1 activity plays an anti-senescent role. When they blocked the gene activity of Sirt1, and thus reduced its protein levels, the effects of supplemental NAD+ on inhibiting ROS generation and BMSC senescence were weakened. Overall, this study confirms that supplemental NAD+ postpones BMSC senescence through Sirt1 signaling.
Can NAD+ Boosting Improve BMSC Clinical Potential?
“Our findings provide a theoretical basis and methodological reference for the widespread application of NAD+ in the field of anti-aging and the acquisition of high-quality BMSCs in [culture] and their better clinical application,” concluded the authors.
These findings have the potential to improve the application of BMSCs cultured in labs and regenerative medicine.

