Scientists Demonstrate NMN Limits Liver Damage with Alcohol Consumption in Mice
A team of researchers identified how nicotinamide mononucleotide (NMN) supplementation affected mice with early-stage chronic alcohol consumption.
A paper published in Human Genomics on December 10, 2019, described how the nicotinamide adenine dinucleotide (NAD+) boosting supplement nicotinamide mononucleotide (NMN) works to treat liver disease caused by alcohol consumption in mice. The team of scientists embarked on this study because chronic alcohol consumption has contributed significantly to liver disease worldwide. The resulting alcoholic liver disease entailed inflammation and dysregulation of metabolism.
NMN Limits Liver Damage in Mice that Ingested Alcohol
The researchers used measurable indicators of a biological state (biomarkers) called alanine aminotransferase (ALT) and aspartate aminotransferase (AST) to determine liver damage in mice given saline (control group) and mice given ethanol (experimental group). As expected, the scientists found significantly higher biomarker levels of ALT and AST levels in mice treated with ethanol (alcohol), which indicated liver damage. The scientists performed an experiment where they gave a group of mice saline and nicotinamide mononucleotide (no alcohol plus NMN) and another group of mice nicotinamide mononucleotide and ethanol (alcohol plus NMN). They found no difference in biomarker levels for liver damage between these groups. These findings suggested that administering NMN limited liver damage in mice that consumed alcohol.
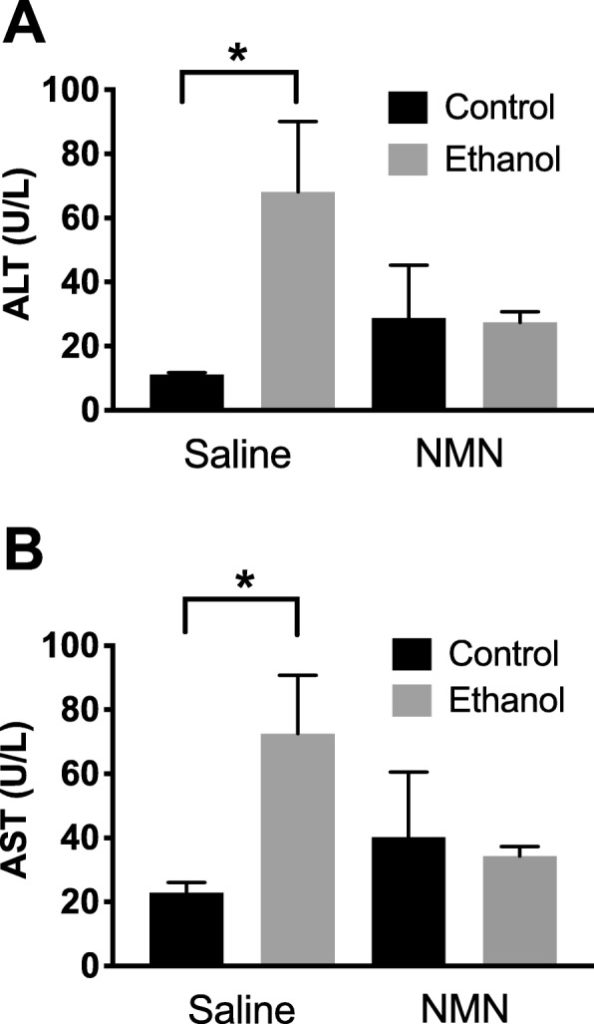
The researchers also wanted to determine if alcohol toxicity decreased levels of available NAD+ in the liver with NMN administration. Previous studies have shown that alcohol toxicity associated with alcoholic liver disease (ALD) facilitated diminished levels of NAD+. Their results provided evidence in this mouse model of ALD showing injection of NMN successfully increased levels of NAD+ with alcohol consumption.
The scientists went on to measure the effects of nicotinamide mononucleotide administration on metabolism in the mouse model of alcoholic liver disease. Previous studies showed that chronic alcohol consumption resulted in dysregulated expression of genes involved in liver metabolism. The studies showed a protein (transcription factor) involved in regulating the expression of metabolic genes, Atf3, had overexpressed levels in liver damage. Alcohol consumption induced overexpression of this protein (Atf3). Such overexpression of this protein (Atf3) inhibited the body’s production of sugars (gluconeogenesis). This study provided evidence NMN administration mitigated these effects in the early stages of chronic alcohol consumption in mice.
The evidence that this study provided showing NMN administration in mouse models of ALD limited liver damage as measured with biomarkers of liver damage may translate to humans. Scientists often have made discoveries using mouse models before applying similar studies in humans. Thus, the possibility remains that the NAD+ booster NMN may act as a therapeutic strategy for intervention in alcoholic liver disease.
Evidence supporting the use of NMN for intervention in alcoholic liver disease came from this study in mice, which showed NMN administration limited liver damage and maintained NAD+ levels with alcohol consumption. Furthermore, NMN may have alleviated metabolic dysfunction associated with chronic alcohol consumption. Further research on this topic was necessary before scientists could make therapeutic recommendations.
Authors of this study included Mohammed A. Assiri of King Saud University, Riyadh, Saudi Arabia; Hadi R. Ali, John O. Marentette, Youngho Yun, Laura M. Saba, Peter S. Harris, and Kristofer S. Fritz of University of Colorado Anschutz, Aurora, Colorado; Juan Liu and Matthew D. Hirschey of Duke University, Durham, North Carolina.
The work and researchers of this study were supported with grants from the National Institutes of Health.

