NAD+ Boosting Drops Treat Age-Related Dry Eye Disease
Japanese scientists have shown that drops containing the NAD+ precursors, NMN or NR, enhance eye gland oily secretions to prevent dry eye disease.
Highlights
- Age-related testosterone decline causes dysfunction of eye glands that produce oily secretions to the eye, resulting in dry eye disease.
- Eye drops with NAD+ boosters NMN or NR ameliorate age-related eye conditions by activating a testosterone synthesizing enzyme in aged mice.
Within our eyelids are tiny glands that keep our eyes moist, and as we age, the production of their oily substance declines, driving a condition called dry eye disease and eye dysfunction. At the same time, testosterone levels decline in meibomian glands. Meibomian gland testosterone levels go hand-in-hand with the oily secretions, showing a link between testosterone decline and age-related dry eye disease and eye dysfunction. Researchers have been searching for ways to boost meibomian gland testosterone levels to improve the production of critical eye-coating oil to prevent evaporative eye disease and loss of eye function.
Published in Nature Aging, Kyoto University researchers show that eye drops with NAD+ precursors NMN or NR at concentrations of 100 uM improve eyelid oil secretion to mitigate dry eye disease in mice. By boosting NAD+ with NMN or NR, meibomian glands increase in size, stimulating the secretion of their oily substance to moisturize eyes. These results illustrate that treating eyes with NAD+ precursors may prevent the progression of dry eye disease during aging.

(Yoshida & Apte 2022 | Nature Aging) Declining NAD+ availability with age contributes to meibomian gland atrophy and an evaporative dry eye. Meibomian gland atrophy stems from the diminished secretion of an oily substance. The production of this oily secretion comes from meibomian gland testosterone production. The enzyme 3β-HSD catalyzes testosterone production from androgen steroid hormone precursors. As we age, NAD+ levels decline, which correlates with reduced 3β-HSD and subsequently reduced testosterone-driven oily eye secretions. The resulting dry eye disorder drives dry eye disease as we age. Topically applied NR or NMN significantly increases NAD+ levels and reverses dry eye disease in mice and may also do so in humans.
Aged Adults Have 20-30% Reductions in Eye Gland Volume
To characterize age-associated eye gland deterioration, Doi and colleagues from Kyoto University examined human eyelid specimens from men and women aged 29-35 and 61-70. The Japan-based research team measured the area of meibomian glands in the upper and lower eyelids and found that the aged glands decreased in size by about 30% in the upper eyelids. These oil-secreting glands shrunk by about 20% in the lower eyelids of the older human participants. These results suggest that eye function deterioration during menopause and andropause may correlate with a functional decline in meibomian glands as illustrated by substantially reduced gland size.
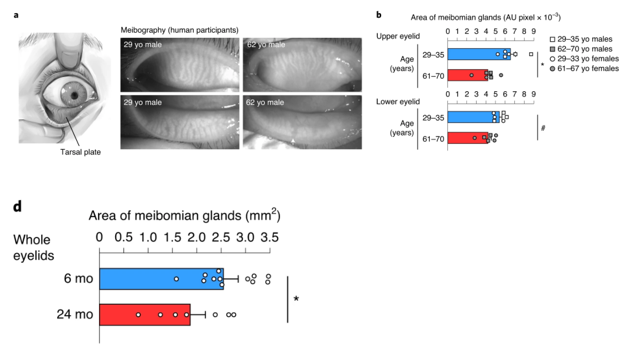
The research team then found a correlation between meibomian gland size reductions and diminished 3β-HSD activity in mice. Compared to six-month-old mice, mice aged 24 months showed a 40% decrease in 3β-HSD enzyme functional capacity. These data indicate reduced meibomian gland size and dysfunction resulting from age-related loss of testosterone production.
To confirm the link between meibomian gland atrophy and diminished oily secretions from the eye (evaporative eye disease), Doi and colleagues examined meibomian gland atrophy in mice. Evaporative eye disease is the predecessor condition that leads to dry eye disease, and the research team found that mice with genetically ablated 3β-HSD had substantial eye gland atrophy from this disorder. Doi and colleagues then concluded that the atrophied eye glands in the absence of the 3β-HSD enzyme drives the onset of evaporative eye disease.
NAD+ Repletion Maintains Meibomian Gland Function
NAD+ repletion has been associated with activating the steroidogenesis enzyme 3β-HSD. In this regard, Doi and colleagues treated the eyes of 18-month-old mice with the NAD+ synthesis inhibitor FK866 in the presence of either NAD+ precursor, NMN or NR. They found that FK866 treatment reduced 3β-HSD activity by approximately 80% but that NMN and NR restored the enzyme’s activation to typical values. Interestingly, NMN improved 3β-HSD 10% higher than baseline activity levels, showing greater effects than NR. Moreover, the presence of FK866 reduced meibomian gland NAD+ levels by about 85%; however, both NMN and NR counteracted these effects and substantially restored NAD+ levels with FK866.
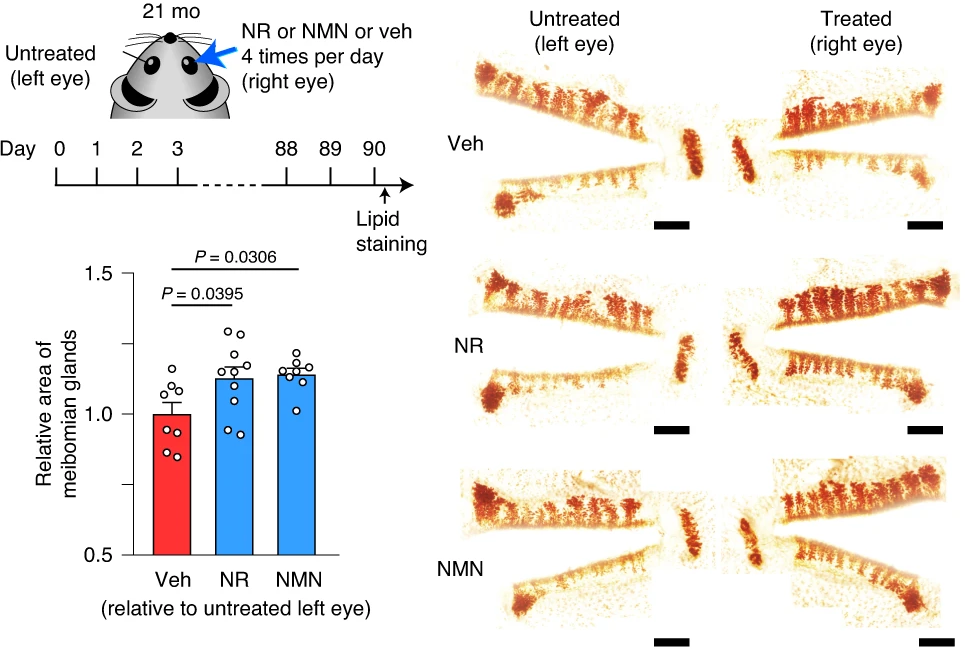
In the same set of experiments, Doi and colleagues found that NMN or NR increased the eye glands’ size by at least 25% in aged, 21-month-old mice. These results were recapitulated with meibomian gland images after 90 days of topical NMN or NR application via eye drops to 21-month-old mice, showing greater structural integrity, which typically translates to improved function. Moreover, NMN or NR eye treatments almost doubled the activation of genes associated with cell proliferation, Wnt2 and Adamts17. Cumulatively, these results point to NMN or NR eye drop treatments possibly preventing or even reversing dry eye disease, at least in mice.
NAD+ Precursor Containing Drops Prevent Aged Eye Dysfunction
“Topical administration of NMN increased NAD+ levels in the meibomian gland,” said Doi and colleagues in their publication. Eye drops containing either NMN or NR were administered to the right eyes of aged mice six times per day for 90 days, which increased endogenous 3β-HSD activation. The resulting improvements were observed with greater meibomian gland integrity. Moreover, the activation of Wnt2 and Adamts17, two genes linked to cell proliferation, increased 20-100% with either NMN or NR treatment. All together, these findings show that eye drops with NMN or NR hold promise for treating dry eye disease.
This captivating research reveals new details regarding NAD+-regulated pathways, highlighting the broad impact of NAD+ on biological activities beyond sirtuins and PARPs. Although prior research on the effects of NAD+ on aging and illness has focused on these well-studied longevity genes, the work presented here encourages us to pursue open-ended techniques to investigate unstudied pathways regulated by NAD+ availability. Lastly, human males and females age differently. For example, women are more susceptible to dry eye disease. The role of testosterone, or lack thereof, in meibomian gland dysfunction described by Sasaki and colleagues could potentially help to explain the sex-related differences in dry eye disease.

