NAD+ and the Sleep-Wake Cycle
NAD+ and its involvement in the sleep-wake cycle (circadian rhythm)
Healthy aging requires maintaining a regular sleep schedule. Dysregulated sleep patterns contribute to poor aging and disease. Moreover, imbalances in genes and molecules involved in the sleep-wake cycle (circadian rhythm) contribute to onset of diseases.
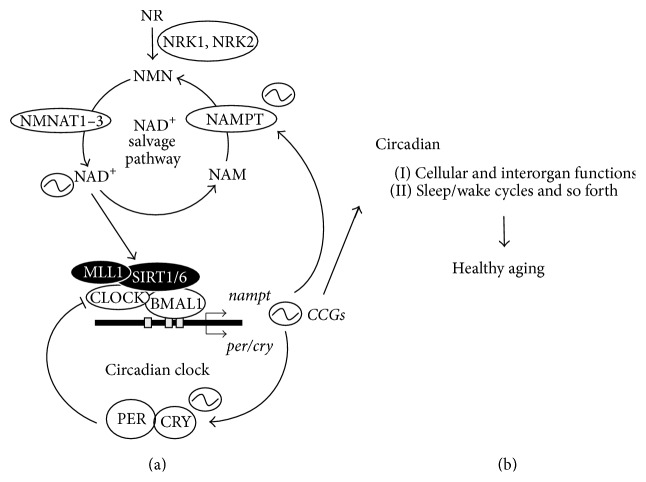
Image from Nakahata & Bessho (2016)
In studies of mice, diseases associated with imbalances in genes and molecules of the sleep-wake cycle include age-related diseases such as cancer, diabetes, and high blood pressure (hypertension) (Nakahata & Bessho, 2016). Accelerated aging and shortened lifespan associates with these imbalances, also (Nakahata & Bessho, 2016). Mice without a gene of the sleep-wake cycle (Bmal1 knockout mice) present with diseases such as sarcopenia, cataracts, decreased subcutaneous fat, organ shrinkage, dyslipidemia, arterial and venous thrombosis, and hypoinsulinaemia and had drastically shortened lifespan (Kondratrov et al., 2007; Nakahata & Bessho, 2016; Shimba et al., 2011). Additionally, mice without another gene of the sleep-wake cycle (clock knockout mice) displayed age-related diseases such as hyperlipidemia, hyperleptinemia, hyperglycemia, and hypoinsulinaemia with shortened average lifespan (Marcheva et al., 2010; Nakahata & Bessho, 2016; Turek et al., 2005). In humans, studies have also linked metabolic disorders like obesity, insulin resistance, heart disease (cardiovascular disease), and cancer to abnormalities in the sleep-wake cycle (Nakahata & Bessho, 2016). Thus, various diseases are linked to imbalances in molecules of the sleep-wake cycle.
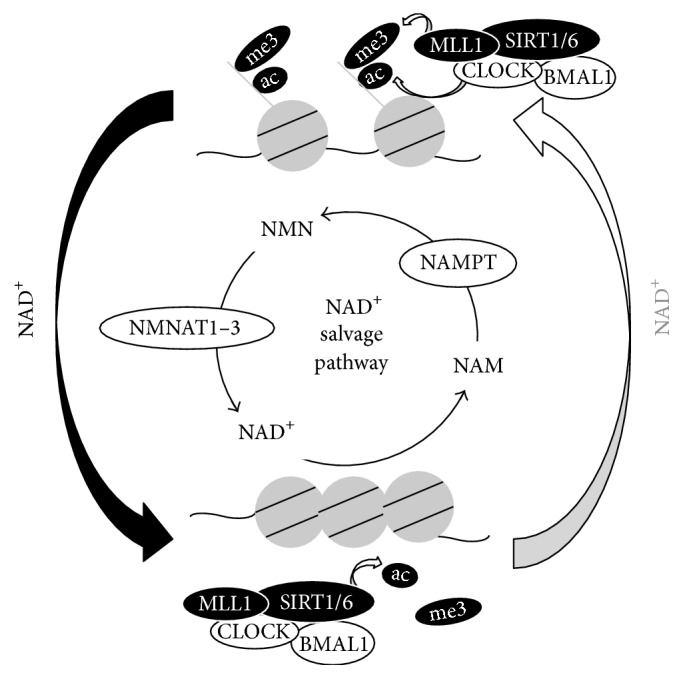
Image from Nakahata & Bessho (2016)
Levels of NAD+ (nicotinamide adenine dinucleotide) oscillate during the sleep-wake cycle. Researchers speculate that NAD+ oscillations during the sleep-wake cycle are reduced or missing in aged animals and humans (Nakahata & Bessho, 2016). Evidence for credulity of this speculation comes from data showing that mice without genes involved in the sleep-wake cycle (circadian rhythm knockout mice) have less NAD+ in their cells compared to normal mice (wildtype mice) (Nakahata et al, 2009; Nakahata & Bessho, 2016; Ramsey et al., 2009). Other research has shown that aged mice have much lower NAD+ in cells compared to younger mice, which suggests that oscillations of NAD+ levels during the sleep-wake cycle could have lower peak levels (amplitudes) in aged animals (Nakahata & Bessho, 2016).
Giving mice molecules that their bodies may synthesize into NAD+ (precursors) has shown to improve age-related diseases and lifespan. Thus, researchers want to find out if these precursor molecules, such as NMN (nicotinamide mononucleotide), may improve NAD+ level oscillations during the sleep-wake cycle to help gene expression and molecule levels of the sleep-wake cycle. If so, these findings would provide evidence that NAD+ levels in the sleep-wake cycle are significant physiologically (Nakahata & Bessho, 2016). This would mean that improving levels of NAD+ during aging could play a role in maintaining healthy sleep patterns. As mentioned, imbalances in the genes and molecules of the sleep-wake cycle are linked with various diseases. Therefore, the possibility remains that improving NAD+ levels during aging may help with molecular health of the sleep-wake cycle.
Figuring Out Whether NAD+ Influences Sleep Patterns and Aging
Accumulating evidence shows the link between molecular balance of the sleep-wake cycle and age-related diseases. NAD+ levels have a strong relationship with the sleep-wake cycle and age-related diseases. No direct evidence has shown that NAD+ acts as the hub between the sleep-wake cycle and age-related diseases. This topic remains for researchers to explore. Figuring out these connections will help to understand aging and therapeutic interventions of age-related diseases.

