Lithium Reduces Senescence and Preserves Kidney Function in Aging Patients
A study published in the Journal of Clinical Investigation identifies an actionable target to delay kidney aging.
Highlights
- Levels of an enzyme called GSK3β increases with age in the kidneys of humans and mice, mainly localizing to kidney cells critical for blood-filtering called podocytes.
- Blocking GSK3β in mice mitigates podocyte senescence – an aging-related phenomenon of cellular arrest in growth and replication – and improves kidney aging.
- Long-term lithium carbonate therapy in patients inhibits GSK3β activity and attenuates kidney cell senescence.
Kidney aging, which predisposes people to a high burden of kidney diseases and systemic comorbidities, is a challenge that continues to grow with increasing life expectancy. Many aging individuals experience a progressive decline in kidney function and alterations. The key feature is the aging of the glomerulus – a cluster of nerve endings, spores, or small blood vessels around the end of a kidney tubule where waste products are filtered from the blood. Since kidney function decreases with age and may soon limit millions of lives, there’s an immense unmet need to develop a pragmatic, actionable target for slowing down kidney aging.
In a study published in the Journal of Clinical Investigation, Fang and colleagues at the University of Toledo College of Medicine found that blocking an enzyme called glycogen synthase kinase 3β (GSK3β) with lithium reduced glomerular aging in mice and humans. These findings should prompt further study of lithium and other GSK3β inhibitors as a means of extending glomerular function in individuals with chronic kidney disease. “Our findings suggest that GSK3β-regulated senescence signaling may be an attractive target for therapeutic interventions aimed at delaying kidney aging,” wrote the authors. “Our findings have significant therapeutic potential because lithium, a standard inhibitor of GSK3β, is an FDA-approved agent for treating affective mental illness.
Senescence is crucial to kidney aging
Many individuals experience a progressive decline in kidney structural integrity and function with age. The key feature of glomerular aging is scarring of the blood-filtering glomeruli (glomerulosclerosis). Glomerular aging involves cellular arrest in the growth and replication (senescence) of various glomerular cells, particularly the terminally differentiated podocytes, a critical constituent of the glomerular filtration barrier. In addition, senescent glomerular cells like podocytes may affect nearby cells in the glomeruli via the signaling of inflammatory signals, which are key drivers of glomerulosclerosis.
Kidney aging is linked to GSK3β levels
By investigating and classifying kidney specimens by age from adults up to 60 years old, Fang and colleagues found a consistent, age-related increase in the percentage of rigid (sclerotic) glomeruli. Signs of age-related podocyte degeneration were also noted, including ultrastructural lesions and reduced levels of gene activity for the podocyte marker Wilms’ tumor 1 (WT-1), indicating podocyte loss. These changes were associated with a modest but significant decline in kidney function.
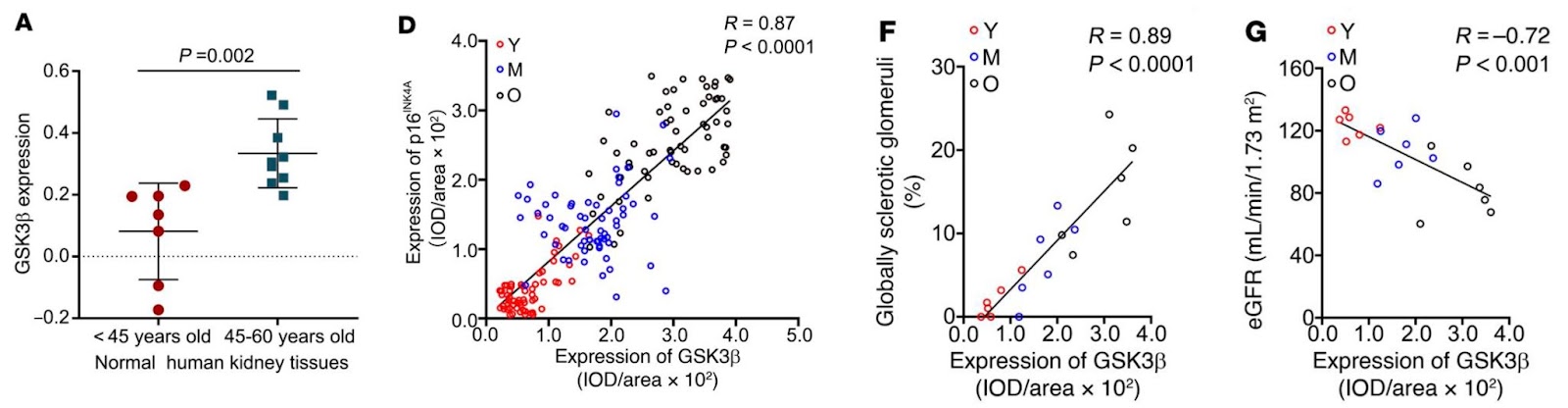
After establishing that the pattern of GSK3β levels in the kidney changed with age in mice, Fang and colleagues next showed that GSK3β increased in parallel with enhanced glomerular and podocyte senescence and the severity of glomerular scarring. Then when they deleted GSK3β in podocytes, features of kidney aging – senescence, podocyte loss, albuminuria, and deteriorating kidney function – and scarring were reduced compared to unaltered mice.
Lithium reduces GSK3β levels and attenuates kidney aging
To target GSK3β-regulated senescence signaling for modifying the kidney aging process, Fang and colleagues treated aging mice with lithium, a known inhibitor of GSK3β. After 3 or 6 months of weekly injections with microdoses of lithium (40 mg/kg) or sodium treatment, lithium but not sodium treated mice had markedly ameliorated signs of kidney dysfunction (albuminuria) and improved kidney function. In addition, lithium treatment significantly attenuated a loss in podocytes and mitigated cellular senescence in glomeruli.
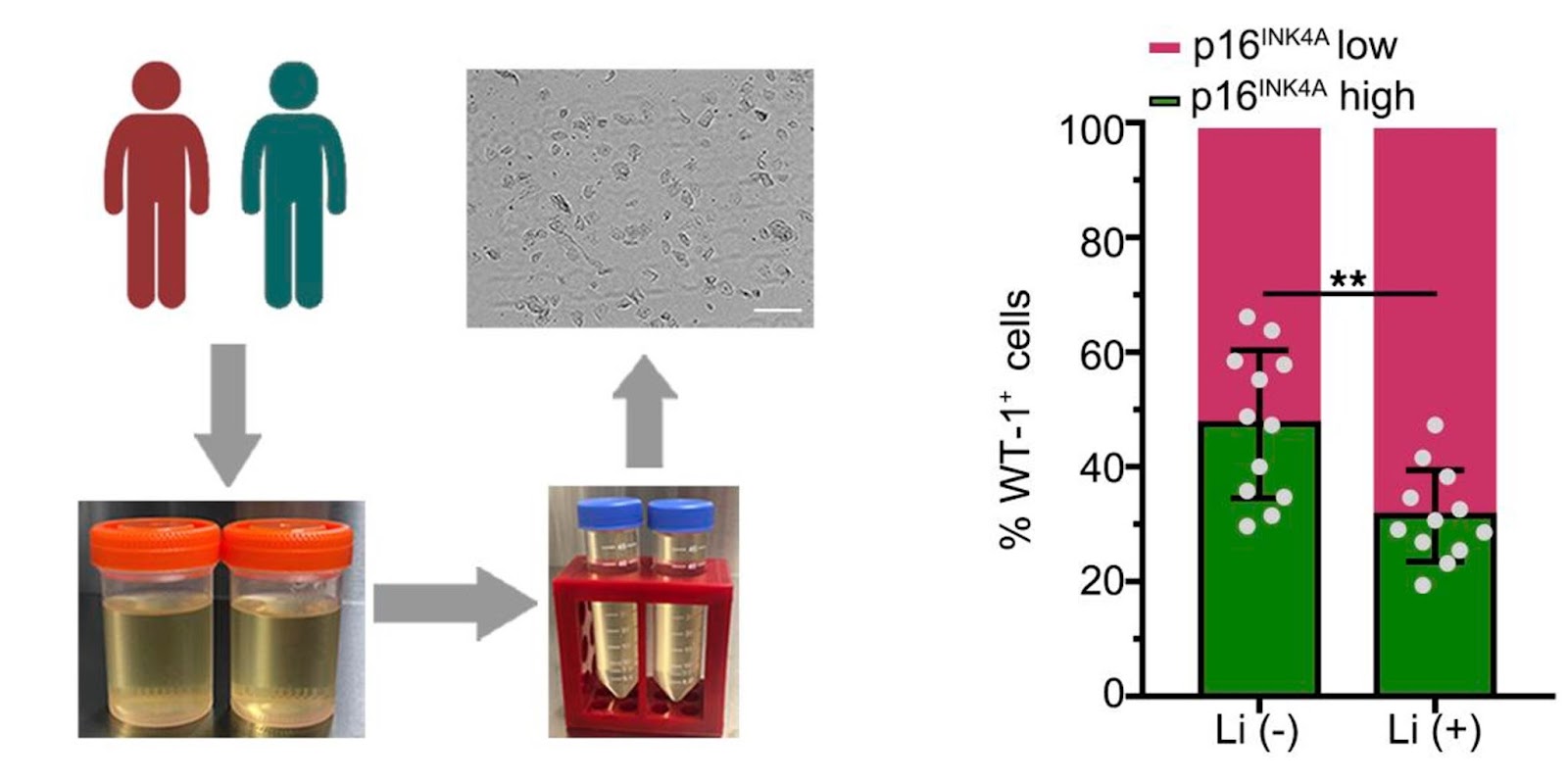
To determine whether lithium alters GSK3β-regulated senescence signaling and kidney aging in humans, Fang and colleagues studied the urine of a group of psychiatric patients who received long-term treatment with lithium carbonate. Since different types of kidney cells are secreted into urine, urine can serve as a non-invasive liquid kidney biopsy for research and diagnostic purposes.
Lithium-treated patients tended to excrete more of the podocytes with an inhibited version of GSK3β and low levels of a senescent marker (p16INK4A), consistent with an anti-aging activity. In parallel, podocyte degeneration was reduced in lithium-treated patients, suggesting that lithium therapy confers a rejuvenating effect on kidney cells, including podocytes. Indeed, lithium-treated patients demonstrated a trend toward better kidney functions.
Lithium as an anti-aging therapeutic
Low-dose lithium has been shown to slow aging in experimental creatures such as roundworms and flies, and epidemiologic research in humans backs this up. Microdose lithium protects against acute kidney infection, chronic kidney disease, and glomerular damage.
Clinical usage of lithium in people with a mental health condition, on the other hand, has been linked to adverse renal effects and clinical outcomes. Nonetheless, most epidemiologic evidence suggests that toxicity to the liver caused by lithium is infrequent and frequently related to high lithium serum levels and extended lithium medication duration.
To attain therapeutic levels in cerebrospinal fluid and cure mental illnesses, substantial dosages of lithium are necessary. As a result, the psychiatric dose of lithium is substantially higher than the amount associated with favorable effects on peripheral organs such as the kidney.
While the ideal lithium dose for kidney protection is uncertain, data shows that a dose less than one-third of the neurobiological dose in animal models is adequate to suppress renal GSK3 activity. This modest dose has considerable impacts on different signaling pathways and kidney aging, as revealed in this and previous research, without noticeable side effects. If validated in large clinical trials, low-dose lithium might also be an effective anti-aging medication for the kidney and, potentially, other organ systems.”

