Latest Study: Repurposed Drugs Prevent Cognitive Decline in Alzheimer’s Disease Model
Temple University researchers find that treating an Alzheimer’s disease mouse model with existing drugs used for altitude sickness and glaucoma prevents cognitive decline and removes sticky Aꞵ proteins in brain vasculature.
Highlights
- Two already FDA-approved drugs, acetazolamide and methazolamide, show a statistical trend toward preventing cognitive decline in an Alzheimer’s disease mouse model.
- The two drugs remove sticky proteins contributing to Alzheimer’s disease in the brain vasculature of Alzheimer’s disease mice.
- Acetazolamide and methazolamide suppress a key enzyme found to have elevated levels in human Alzheimer’s patients’ mitochondria — carbonic anhydrase VB.
The age-associated disease with profound adverse effects on memory, Alzheimer’s disease, encompasses the accumulation of sticky proteins called Aꞵ deposits in the brain’s blood vessels. Researchers have prioritized the removal of these protein deposits in hopes of improving cognition and reversing the disease’s course. Furthermore, identifying drugs already approved by the FDA that alleviate cognitive decline in Alzheimer’s disease is an attractive proposition since such drugs have already gone through approval processes.
Published in Alzheimer’s & Dementia, Fossati and colleagues from Temple University in Philadelphia present data showing that repurposing the glaucoma and altitude sickness drugs acetazolamide and methazolamide prevent cognitive decline in an Alzheimer’s mouse model. The researchers also show that the Alzheimer’s disease model mice treated with the drugs undergo the elimination of sticky proteins that purportedly contribute to Alzheimer’s disease — Aꞵ deposits — in the brain vasculature. These findings suggest that repurposing acetazolamide and methazolamide against Alzheimer’s disease may reverse the disease’s course.
Repurposed Drugs Prevent Cognitive Decline and Reduce Sticky Protein Buildup in the Brain
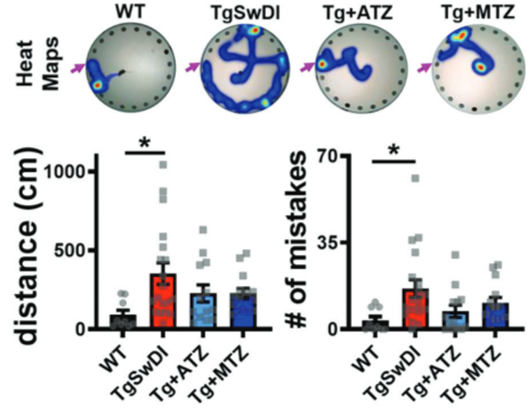
Because severe memory decline goes with Alzheimer’s disease, Fossati and colleagues measured Alzheimer’s disease model mice’s cognition in a test of learning and memory — the Barnes maze. Mice have a natural aversion to open spaces, so the Barnes maze capitalizes on this aversion by having mice start in the center of a circular surface. Twenty circular holes line the outer banks of the central surface onto which the mouse is placed, and within one of the holes is an escape box. The mice learn the location of the hole with the escape box by associating it with visual cues, like objects and colors, around the maze. Alzheimer’s model mice placed in the Barnes maze covered about five times the distance and made about five times the mistakes that healthy mice made to find the escape box, an indicator of impaired memory and overall cognition. Acetazolamide and methazolamide treatments cut the Alzheimer’s model mice’s distance covered and the number of mistakes in finding the escape box in half. Although acetazolamide and methazolamide didn’t significantly improve cognition in Alzheimer’s model mice, as indicated by improved distances and mistake numbers, a statistical trend toward improved cognition was associated with their treatments.
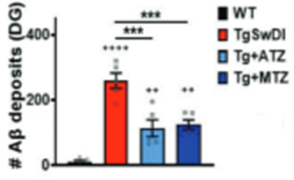
Since the accumulation of sticky Aꞵ deposits are associated with and thought to contribute to Alzheimer’s disease, Fossati and colleagues measured their abundance in Alzheimer’s disease model mice treated with the two repurposed drugs. They found that acetazolamide and methazolamide more than cut the Aꞵ deposit abundance in half within brain blood vessels. These data suggest that the two repurposed drugs prevent cognitive decline in Alzheimer’s disease model mice by eliminating harmful Aꞵ protein deposits in the brain’s vasculature.
“Both acetazolamide and methazolamide were highly effective in reducing amyloid deposition and in improving cerebrovascular function,” Dr. Fossati said in a press release. “Our behavioral studies showed that, as Alzheimer’s pathology decreased, [acetazolamide or methazolamide]-treated mice experienced noticeable gains in cognitive function.”
The Identification of a Key Enzyme Possibly Contributing to Alzheimer’s Disease
The study uncovered a specific enzyme, carbonic anhydrase VB, that exhibits higher levels in Alzheimer’s disease human patients’ cellular powerhouses, the mitochondria. Acetazolamide and methazolamide reduce this enzyme’s function throughout the whole cell in the Alzheimer’s disease mouse model. Since elevated carbonic anhydrase VB levels were found in Alzheimer’s disease patients’ mitochondria, Fossati and colleagues want to target this enzyme specifically in the mitochondria with new drugs in the future. Targeting mitochondria-specific carbonic anhydrase VB may enhance the anti-Aꞵ deposit and cognitive function improving effects of the drugs.
“The therapies we used for the current study target carbonic anhydrases in the whole cell,” Dr. Fossati explained in the press release. “If we can target the enzyme specifically within mitochondria, the efficacy of therapy could improve greatly, and side effects could be reduced.”
The next step in repurposing these drugs for use in human Alzheimer’s disease patients entails clinical trials. According to a press release from Temple University, such clinical trials could be underway soon.
A substantial limitation to the study comes from Fossati and colleagues not measuring the new drugs’ effects on protein conglomerates in the brain — tau neurofibrillary entanglements. These neurofibrillary entanglements typically accompany Alzheimer’s disease along with Aꞵ deposits. Future studies, namely the potential repurposed drugs’ clinical studies, should examine how acetazolamide and methazolamide affect tau accumulations in patients with Alzheimer’s disease.
Model: TgSwDI mice
Dosage: 20 mg/kg per day of acetazolamide or methazolamide in food for eight months

