Innovative Drug Improves Vision In Blind People
CRISPR gene therapy improves the vision of people with hereditary blindness.
Highlights:
- CRISPR gene editing technology was used to improve the eyesight of individuals with a rare disease caused by a genetic mutation that leads to vision loss and blindness.
- The CRISPR drug was designed to remove said genetic mutation, allowing the eye’s light-detecting cells to function properly.
- Before the FDA approves the new CRISPR drug, more funding will be needed to conduct a larger clinical trial.
For the first time in history, a gene editing technology called CRISPR was tested on people with a rare inherited retinal degenerative disorder. In the Phase 1/2 clinical trial, a drug called EDIT-101 was injected into one eye (the worse eye) of 14 participants with Leber congenital amaurosis (LCA). Remarkably, after a few months, some of the participants saw improvements in vision, including Oliva Cooke and Michael Kalberer.
Oliva, a 22-year-old college student, told CNN the gene therapy allowed her to see faces. During the holidays, at dusk, Oliva was with friends on a balcony wrapped in Christmas lights. “With my right [untreated] eye, I was not able to see their facial features,” said Oliva. “I was only able to see their silhouette. With my left eye, I could see everything on their face.”
Michael told CNN that the therapy allowed him to see color. He said, “It was a pretty cool moment to see strobe lights on the dance floor of my cousin’s wedding change color.” The 46-year-old added that he could normally only see shadows and flickering lights but not colors. However, Michael still can not see text or photos on his phone, saying “My disease is still here… I’m not cured.”
How The Gene Therapy Works
Oliva and Michael were born with a mutation in a single gene, called CEP290. The CEP290 gene provides the instructions for a protein found in many cells, including our eye’s light-detecting cells — photoreceptors. Our photoreceptors are housed within our retinas — a thin layer of tissue covering the back of our eyes — and without them, we would not be able to see. It follows that people harboring Oliva and Michael’s CEP290 mutation experience significant vision loss and potential blindness due to dysfunctional photoreceptors.
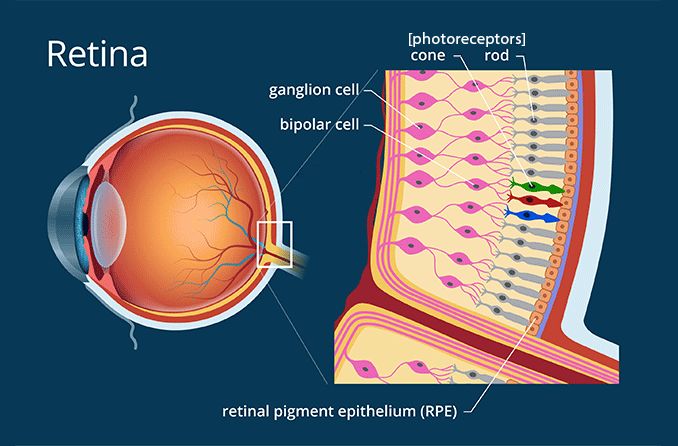
When it comes to diseases caused by a single gene mutation, CRISPR is the perfect choice. CRISPR was discovered in 1987 in E. coli and has since been turned into a gene editing technology that can remove mutations from our DNA. EDIT-101 is a CRISPR drug specifically designed to target the CEP290 mutation that induces retinal degeneration. By removing the CEP290 mutation, EDIT-101 can improve the function of photoreceptors, leading to better vision.
Larger More Expensive Study Needed for FDA-Approval
The variation of LCA treated in the study, called the BRILLIANCE trial, affects about 1,500 people in the US. Furthermore, the treatment may be more effective in patients with two, rather than one copy, of the CEP290 mutation, leaving only about 300 affected Americans. For this reason, the study’s funder Editas Medicine has decided not to progress the study alone and is seeking collaborators to initiate a Phase 3 clinical trial. Phase 3 clinical trials generally include hundreds of participants, making them more expensive to conduct than Phase 1 or Phase 2 trials.
“The results from the BRILLIANCE trial provide a proof of concept and important learnings for our inherited retinal disease programs. We’ve demonstrated that we can safely deliver a CRISPR-based gene editing therapeutic to the retina and have clinically meaningful outcomes,” said Edita’s CEO Gilmore O’Neill in a press release. “While we will not progress EDIT-101 on our own and have made the decision to pause enrollment, we have the patient community top of mind and are looking for a collaboration partner to advance this program.”
If EDIT-101 proves to be a success in a Phase 3 clinical trial, it may eventually receive FDA approval. This would not be unprecedented, as a gene therapy called Luxturna was approved by the FDA for the treatment of another variant of LCA caused by a mutation in the RPE65 gene. However, Luxturna’s mechanism does not involve the removal of the RPE65 mutation with CRISPR. Instead, Luxturna utilizes a delivery system called adeno-associated virus (AAV) to insert a functional RPE65 gene into cells.
Targeting Aging with Gene Therapy
Aging is a complex process that scientists are still trying to understand at the cellular and molecular level. Still, several genes in humans have been found to be associated with aging. Therefore, it is possible that removing pro-aging genes with CRISPR and inserting longevity genes with CRISPR or AAV gene therapies could ameliorate the ravages of aging at the biological level.
Recently, a gene therapy for VEGF — a gene that promotes the growth of new blood vessels — was shown in a Phase II trial to improve the exercise capacity of individuals with coronary artery disease while also alleviating symptoms. As VEGF has been shown to increase the lifespan of mice, this study demonstrates that inserting longevity genes into humans is feasible. These promising results may lead to a flurry of new gene therapies that counteract chronic diseases associated with aging.

