Neuronal Stimulation Extends Lifespan by Activating NMN Synthesis
Activating specific neurons improves the release of a nicotinamide mononucleotide (NMN)-synthesizing enzyme — eNAMPT — from fat tissue and modestly extends lifespan in mice.
Highlights
- Aging dysregulates neurons within a brain region called the hypothalamus, impeding fight-or-flight signals to fat tissue.
- Stimulating these neurons improves physical activity and modestly extends lifespan in mice through the release of an NMN-synthesizing enzyme (eNAMPT) from fat tissue.
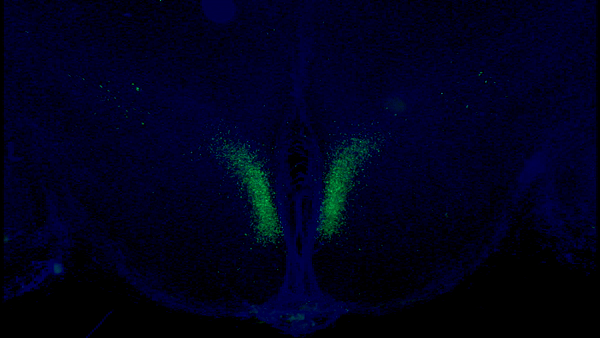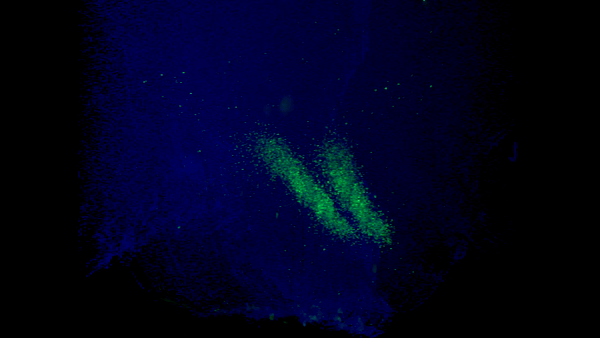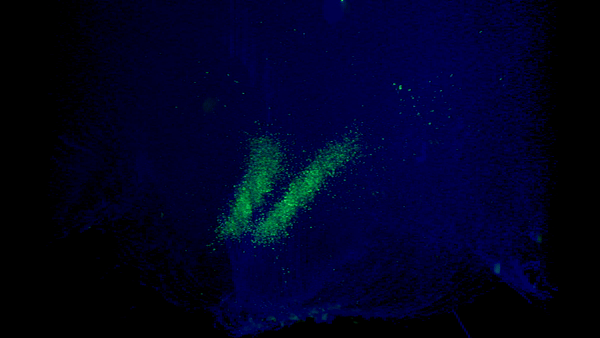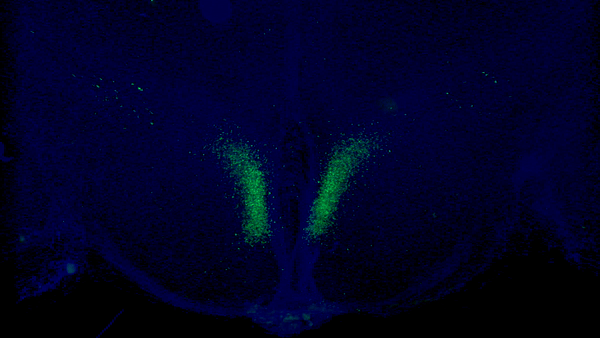
A seminal study published in Cell Metabolism from Imai and colleagues at Washington University in St. Louis shows that stimulating a specific type of neuron in the hypothalamus modestly extends mouse median lifespan by ~7%. In this study, the researchers demonstrate that a protein within these neurons — Ppp1r17 — loses its signaling function with age, which impairs nervous system communication with fat tissue.
This impaired communication between the brain and fat tissue facilitates increased fat mass and reduced physical activity in mice. Through activation of these neurons, Imai and colleagues increased physical activity and the release of the NMN-synthesizing eNAMPT enzyme from fat tissue. These findings point to targeting a specific set of neurons in the hypothalamus, which may play a key role in regulating how we age.
In mammals, the hypothalamus and its communication with other tissues play a key role in aging. Recently, research has demonstrated that hypothalamic neurons possess the NMN transporter — Slc12a8 — to regulate energy metabolism and skeletal muscle function. In fact, through increasing the abundance of the NMN transporter in this brain region, Imai and colleagues were able to counteract an age-related decline in muscle strength in mice. These findings support that the communication between the hypothalamus and other tissues like muscle or fat is crucial to counteract aging and promote lifespan.
Increasing Hypothalamic Neuron Activity Increases Physical Activity and Extends Lifespan
In one of their previous studies, Imai and colleagues found a specific population of neurons in the hypothalamus that are key in aging and longevity. They sought to identify subgroups of these neurons by measuring their gene activity. They found one specific subset of neurons in a part of the hypothalamus called the dorsal medial hypothalamus (DMH) with more than a 15-fold increase in gene activity for the protein Ppp1r17, thus naming them DMHPpp1r17 neurons.
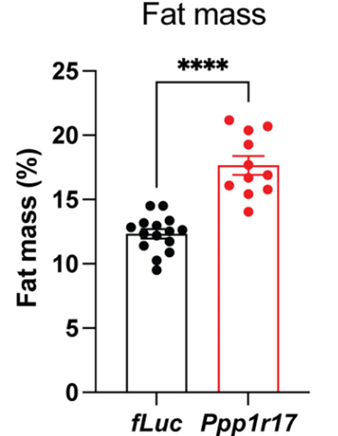
Through genetic engineering, Imai and colleagues reduced Ppp1r17 protein levels within these neurons in mice and found that the mice exhibited about ~30% increased fat mass. The researchers also found dramatically reduced connections to fat tissue from the sympathetic nervous system, which modulates the fight-or-flight response. These findings suggest that DMHPpp1r17 neurons communicate with and regulate fat tissue function through Ppp1r17 protein function, primarily by activating the sympathetic nervous system responsible for the fight-or-flight response.
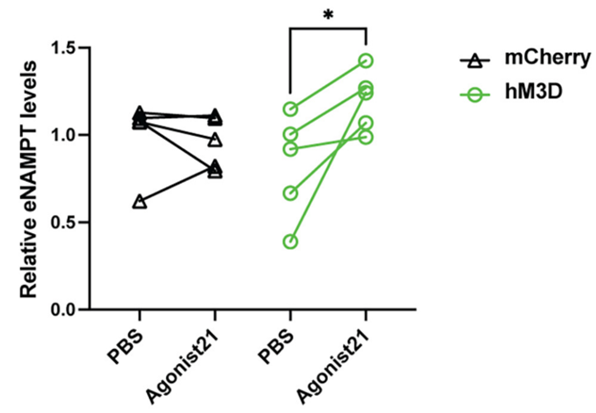
Because Imai and colleagues observed reduced Ppp1r17 protein signaling and decreased DMHPpp1r17 neuron activity during aging, they sought to find whether activating these neurons could alleviate fat tissue dysfunction. To do so, they examined the effects of activating these neurons on the release of eNAMPT. The Washington University-based researchers genetically engineered mice so that they could specifically induce the activation of DMHPpp1r17 neurons. After activating the neurons, they found that the release of eNAMPT from fat tissue significantly increased. Moreover, activating these neurons increased mouse wheel running activity. These findings suggest that activating these neurons in the hypothalamus alleviates fat tissue dysfunction in aged mice.
Since the hypothalamus is well-known as a control center for aging, Imai and colleagues measured whether activating DMHPpp1r17 neurons extends mouse lifespan. Interestingly, activating these hypothalamic neurons extended the median lifespan by ~7% in these mice. These results would translate to about an additional five extra years of life for an adult human who lives to age 75. These findings suggest that improving the crosstalk between the brain and fat tissue via activating DMHPpp1r17 neurons could serve to improve aging.
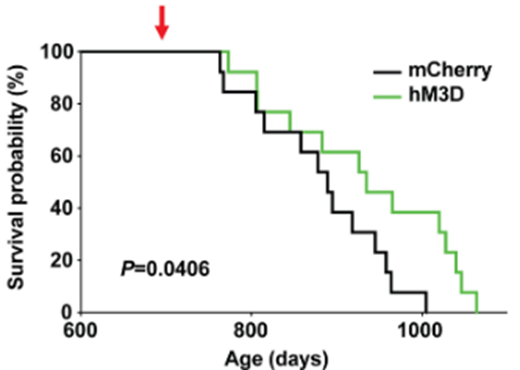
(Tokizane et al., 2024 | Cell Metabolism) Activating DMHPpp1r17 neurons in the hypothalamus increases median and maximum lifespan by ~7% and 20%, respectively, in mice. Activating DMHPpp1r17 neurons in genetically engineered mice (hM3D; green bar) to activate increases median lifespan by ~7% and maximum lifespan by ~20% compared to non-genetically engineered mice (mCherry; black bar).
Maintaining Crosstalk Between the Brain and Fat Tissue with eNAMPT
The study’s principal investigator, Shin-Ichiro Imai, suggests that one possible way to maintain the crosstalk between the brain and fat tissue is to administer eNAMPT. eNAMPT is the NMN-synthesizing enzyme produced in fat tissue that returns to the brain, which could fuel the hypothalamus to activate DMHPpp1r17 neurons. When released from fat tissue, this enzyme is packaged into microscopic compartments called extracellular vesicles (hence, “e” in eNAMPT) that can be isolated and collected from blood.
“We can envision a possible anti-aging therapy that involves delivering eNAMPT in various ways,” says Imai in a press release. “We already have shown that administering eNAMPT in extracellular vesicles increases cellular energy levels in the hypothalamus and extends life span in mice. We look forward to continuing our work investigating ways to maintain this central feedback loop between the brain and the body’s fat tissues in ways that we hope will extend health and life span.”
This begs the question of when administering eNAMPT will be available for humans. eNAMPT is currently unavailable for human consumption, and administering eNAMPT has only been used experimentally in animal models like rodents. However, for anyone interested in supplementing with NMN, the NAD+ precursor synthesized by eNAMPT, it is readily available for online purchase. Nonetheless, it remains uncertain if NMN supplementation offers the same hypothalamus-stimulating benefits as administering the eNAMPT enzyme itself.
Model: C57BL/6J male mice
Dosage: Ppp1r17-Cre mice were intraperitoneally injected with 0.5 mg/kg of Agonist 21 for eight days

