Cornell Researchers Show Unequal Distribution of NAD+ Across Organs Following NMN Administration
NMN significantly boosts NAD+ in liver, kidney, heart, intestine, lung, pancreas, and fat tissue in mice with wide variation dependent on the route of administration.
Highlights:
- Without NMN, NAD+ levels are highest in the kidney and liver of young mice and lowest in blood and fat tissue.
- NMN, administered either orally or injected, raises liver NAD+ levels more than in other tissues.
- The majority of NMN is converted to other NAD+ precursors (i.e. nicotinamide or nicotinamide riboside [NR]) before being converted to NAD+.
Despite extensive research on NAD+ precursors like NMN, just how and where NMN is distributed throughout the organs of the body to elevate NAD+ content remains poorly understood.
Now, researchers from Weill Cornell Medicine report in the International Journal of Molecular Sciences that NAD+ is unevenly distributed across different organs and tissues in young mice. They also show that NAD+ is elevated to differing degrees in response to NMN, depending on the route of administration. Furthermore, they show that most NMN is converted to nicotinamide or nicotinamide riboside (NR) before being converted to NAD+.
Single-Dose NMN Raises NAD+ Predominately in the Liver
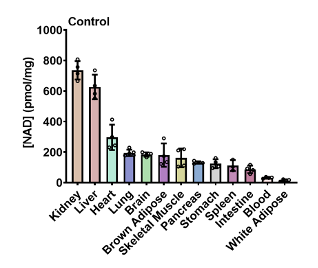
Sauve and colleagues first determined the concentration of NAD+ in various organs and tissues before the administration of NMN in young mice. They found that NAD+ was highest in the kidney and liver, followed by the heart. NAD+ concentrations were lowest in the blood and fat tissue. Overall, the NAD+ concentrations across tissues varied widely — up to 30-fold — demonstrating the unequal distribution of NAD+ across organs.
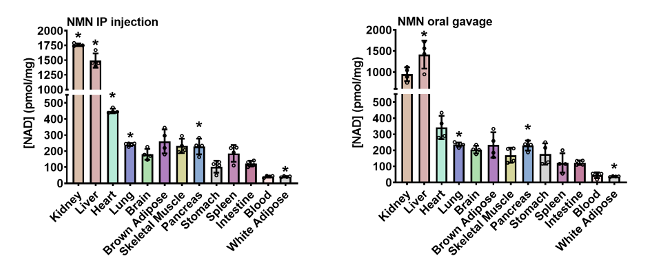
It may be more practical to intake NMN orally, such as in capsule form, yet intravenous injections are still available. Moreover, in animal studies, NMN is usually administered either orally or via injections. Therefore, Sauve and colleagues administered 500 mg/kg of NMN to young mice via both routes. Specifically, by abdominal cavity (IP, intraperitoneal) injections, or via a force-feeding method called oral gavage.
Four hours after IP injections, NAD+ increased approximately 2.4-fold in the liver and kidney while fat tissue NAD+ increased about 2-fold. Significant elevations in NAD+ were also observed in pancreas, heart, and lung tissue. In contrast, oral gavage was less effective, except for in the liver which showed a 2.3-fold increase in NAD+. NAD+ was also boosted in the kidney, fat tissue, pancreas, and lung after oral gavage. Contradicting other studies, NAD+ was not shown to be elevated in skeletal muscle after a single dose of NMN, suggesting long-term administration may be required.
To evaluate how NMN is metabolized into NAD+ across different tissues and organs, Sauve and colleagues radioactively labeled three different portions of the chemical structure of NMN. Each label corresponded to a different metabolic pathway, allowing the researchers to determine the steps taken by NMN to be synthesized to NAD+. The results showed that the majority of NMM is broken down to other NAD+ precursors — either nicotinamide or nicotinamide riboside (NR) — before finally becoming NAD+.
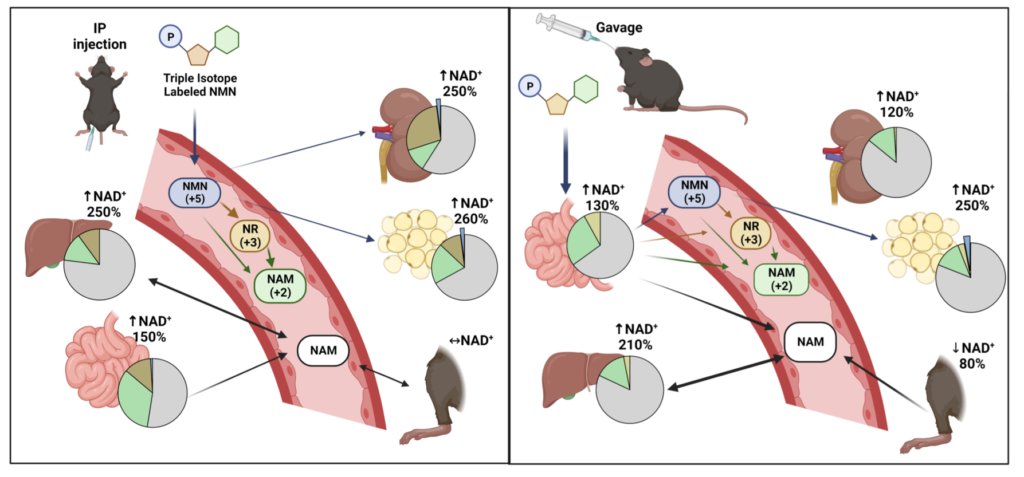
Overall, the findings of Sauve and colleagues reveal that the tissue and organ distribution of NAD+ elevations is markedly varied in response to a single dose of NMN. Additionally, not much of the NAD+ synthesized from NMN comes from the direct conversion of NMN to NAD+. Rather, much of the NMN is first converted to nicotinamide or NR before being converted to NAD+. How this pertains to the effectiveness of raising tissue NAD+ levels with NMN over these precursors is unclear.
Study Limitations
A major limitation of the study is that Sauve and colleagues only examined young mice. A future study under the same conditions but with older mice may help to understand how NAD+ levels change with age across different organs and tissues. Another major limitation is that only a single dose of NMN was tested. In most studies, NMN is provided to animals or humans over the course of weeks or months. Therefore, the distribution of NAD+ could change drastically over time if NMN enters the bloodstream chronically. Thirdly, due to practical limitations, not all muscle groups were analyzed, and neither was bone, both important therapeutic targets for NMN. Still, the results shown by Sauve and colleagues provide a good starting point for these future studies.
Model: 10-week-old male C57BL/6 mice
Dosage: 500 mg/kg of NMN

