Brad Stanfield and Matt Kaeberlein’s New Rapamycin Aging Clinical Trial Measuring Strength and Endurance
Led by Brad Stanfield, MD, and featuring Matt Kaeberlein, PhD, the latest rapamycin anti-aging trial will test the effects of rapamycin supplementation on strength and endurance in older adults.
Highlights:
- Strength and endurance will primarily be measured by how many times participants can stand up from and sit down on a chair for 30 seconds.
- Walking speed and grip strength will also be measured, along with quality of life and various blood markers of aging, including an inflammatory marker.
- The hypothesis is that rapamycin will deter age-related muscle atrophy and provide a synergistic effect when combined with exercise.
As published in BMC, a new double-blind, randomized, placebo-controlled clinical trial will test the effect of weekly rapamycin on muscle strength and endurance in older adults. The study will help deny or confirm the legitimacy of rapamycin — the most potent life-extending compound in mice — as a proper anti-aging compound for humans.
The trial will enroll 40 participants aged 65 to 80. They will receive either 6 mg/week of rapamycin (sirolimus) or a placebo pill for 13 weeks. Moreover, the participants will engage in a home exercise program to promote improvements in muscle strength and endurance. Strength and endurance will be tested by the 30-second Chair-Stand Test, whereby participants will see how many times they can sit down on and stand up from a chair in 30 seconds.
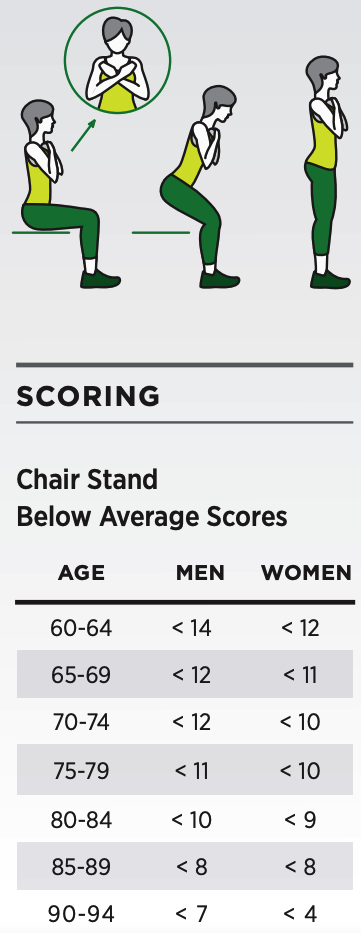
Changes in muscle strength and endurance will also be measured by the 6-minute walking test and handgrip strength. The 6-minute walking test assesses endurance by measuring how far each participant can walk in 6 minutes. Handgrip strength, usually measured with a hand-held dynamometer, can estimate strength, an indicator of longevity. Additionally, each participant will be asked 36 questions to determine their quality of life with the 36-Item Short Form Survey (SF-36).

Furthermore, blood samples will be taken from the participants to analyze various markers of organ and cell health. This will include kidney function (urea and electrolytes), “liver function tests,” average blood glucose (HbA1C), cardiovascular function (lipids, such as LDL cholesterol), endocrine/hormone function (IGF-1), and inflammation (hs-CRP). DNA methylation will also be measured to determine biological age. These blood markers may be useful in assessing the effect of rapamycin on body-wide aging.
Finally, safety and tolerability will be monitored to help confirm the feasibility of older adults taking rapamycin as an anti-aging supplement.
The Promise of Rapamycin
As we age, our muscles shrink and we lose strength at a rate of 1% per year after the age of 40, making it difficult to move. A lack of mobility from weak muscles also leads to a loss of independence and lower quality of life. Moreover, muscle strength decline is associated with an increased risk of early death. While resistance exercise and adequate protein intake can mitigate muscle loss with age, rapamycin may provide an extra boost.
Rapamycin is approved by the FDA (Federal Drug Administration) to prevent organ transplant rejection by suppressing the immune system. It is also prescribed as an anti-cancer drug. It is a direct inhibitor of a protein complex called mTORC1, a nutrient-sensing complex. The dysregulation of mTORC1, namely its chronic activation, is associated with age-related diseases and is considered an underlying driver of aging.
When administered during middle age, rapamycin has been shown to prolong the lifespan of mice by up to 60%. It has also been shown to improve bone and heart health in mice. In rats, rapamycin reduces body weight, increases grip strength, and improves running distance. Additionally, previous clinical trials have shown that rapamycin is safe and well-tolerated by older adults. Moreover, a recent pilot study suggested that rapamycin could increase muscle mass in older women.
The rationale behind this latest rapamycin clinical trial is based on a study showing that chronic activation of mTOR leads to muscle atrophy. The atrophy is primarily due to a reduction in autophagy — the system our cells use to get rid of unwanted deleterious material, like broken proteins. The hypothesis is that by inhibiting mTOR with rapamycin, atrophy can be counteracted. Moreover, combining weekly rapamycin supplementation with exercise may provide a synergistic effect on muscle growth, or at least muscle loss prevention.
When Will the Study’s Results Be Published?
The study was funded by physician and YouTuber Dr. Brad Stanfield through the sale of his own multivitamin supplement, and a life sciences company called Vitasang. In a recent video, Dr. Stanfield was excited to share a recent email he received stating that participant recruitment was finished. He says that data collection should be done by January of next year (2025), at which time the data will be analyzed. Since it can take nearly two years to get a manuscript published after a clinical trial’s completion, we may see the results in a peer-reviewed journal by 2026. However, Dr. Stanfield may divulge the results of the study on his YouTube channel.

