Study Proposes Prevention Strategy for Multi-Organ Fibrosis
The NAD+ consuming enzyme CD38 is a potential therapeutic target for tissue scarring autoimmune disorders
Highlights
- An NAD+ consuming enzyme CD38 is elevated in an autoimmune disorder called systemic sclerosis
- Increased levels of CD38 are linked to augmented scarring and thickening of tissue in fibrotic responses
- NAD+ boosting prevents multi-organ fibrosis
Our understanding of the causes of autoimmune disorders like systemic sclerosis — where the immune system attacks the body, leading to organ scarring called fibrosis — remains unclear. We know that age-related injury is linked to the decline in levels of nicotinamide adenine dinucleotide (NAD+), a molecule critical to cell function and vitality. Research has pointed towards CD38, an NAD+ consuming enzyme, as playing a major role in imbalanced NAD+ levels. But whether C38 is a major player in multi-organ fibrosis of autoimmune disorders has not been shown.
For the first time, Shi and colleagues show that CD38 levels are increased in patients with the autoimmune disorder systemic sclerosis of the skin. The levels of CD38 levels in the skin associate with molecular fibrosis signatures and clinical fibrosis scores, while levels of key enzymes that synthesize NAD+ are unaltered. And when they boosted NAD+ levels by shutting down CD38 or supplementing with NAD+ precursors, mice became protected from fibrosis of the skin and lung.
“These results open the door to entirely novel treatments for fibrosis and scleroderma. Using precision medicine, these treatments could be selectively targeted to block CD38 in scleroderma patients who have elevated CD38,” says study author Bo Shi, Ph.D., a research assistant professor of dermatology at Northwestern Medicine.
CD38 is central to NAD+ breakdown
The enzyme CD38 sits on the outer surface of both immune and certain non-immune cells. This NAD+ consumer typically faces the outside of the cell, although it is also present on intracellular structures with one or more specific jobs in the cell called organelles, including the nucleus and mitochondria. As the main enzyme eating up NAD+ in mammalian tissues, CD38 plays a key role in the age-dependent decline in NAD+. It is, therefore, not surprising that tissue levels and activity of CD38 are negatively correlated with NAD+ levels during aging, and that inhibiting CD38 preserves tissue NAD+.
Is CD38 the missing link in autoimmune fibrosis?
Enzymes dependent on NAD+ called sirtuins, which are known for their role in aging and longevity, have been shown to play a key role in regulating fibrotic responses. Moreover, researchers have shown impaired sirtuin levels and activity in fibrotic skin and lungs from patients with systemic sclerosis, implicating sirtuin dysregulation in pathogenesis. But what is causing sirtuin dysregulation in patients with systemic sclerosis and whether CD38 is involved is currently unknown. CD38-induced NAD+ level reductions may inhibit NAD+-dependent sirtuin activity.
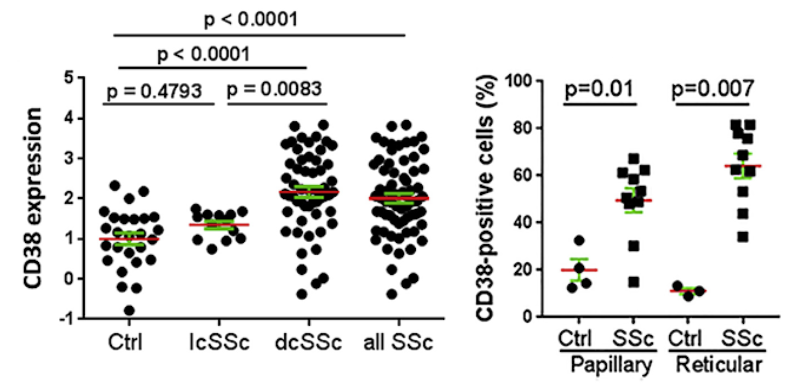
Fibrosis severity is linked to CD38 levels
Shi and colleagues now show that CD38 is substantially elevated in the skin in patients with systemic sclerosis, the prototypic multisystem fibrotic disease. Levels of CD38 are associated with both clinical disease severity and profibrotic signaling activity, while the expression of key synthesizing enzymes in the NAD+ salvage pathway is unaltered compared to healthy controls. Similar alterations in tissue CD38 seen in a mouse model of scleroderma were accompanied by a significant reduction in systemic NAD+, reflecting a conserved linkage between disrupted NAD+ homeostasis and fibrosis in both systemic sclerosis (SSc) and mouse models of disease.
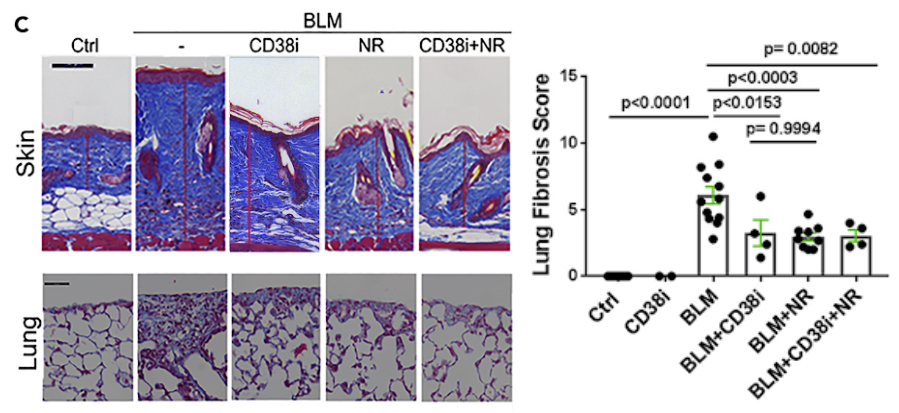
Maintaining NAD+ levels attenuates fibrosis
Blocking CD38 in mice by either genetic targeting or treatment with a selective NADase inhibitor alone or combined with NAD+ precursor supplementation resulted in boosting NAD+ levels and reduced fibrosis propensity in multiple organs. Fibrotic responses were also attenuated in explanted fibroblasts lacking CD38. Similarly, boosting organismal NAD+ via dietary supplementation with the precursor nicotinamide riboside (NR) prevented fibrosis in the skin and lungs.
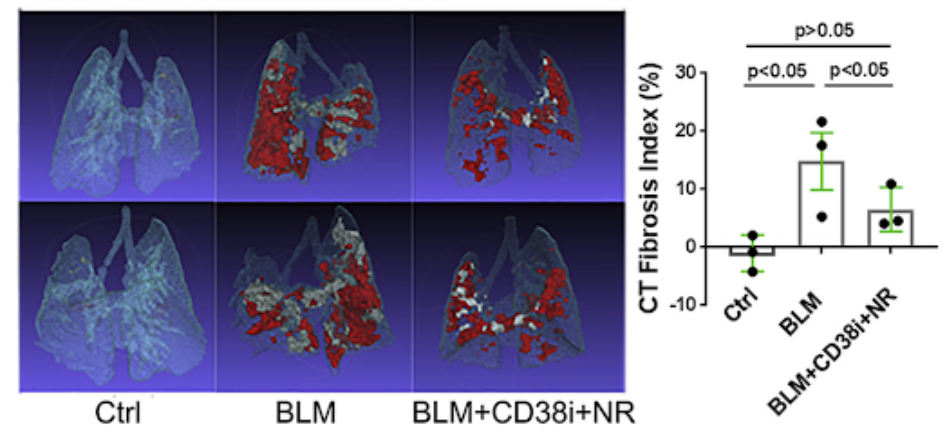
A new therapeutic target to treat chronic fibrosis
“We’ve defined a pathway that for the first time mechanistically links inflammation and metabolism to scarring in system sclerosis,” says study author John Varga, M.D., division chief of rheumatology at Michigan Medicine. “These results provide a major advancement in unraveling the complexities of the disease, which is chronic, progressive, and potentially deadly for many affected.”
Together, these observations uncover a previously unsuspected fundamental role for CD38-mediated NAD+ metabolism in systemic sclerosis fibrosis. CD38 has a previously unrecognized pathogenic role in multiple organ fibrosis via dysregulation of NAD+ homeostasis. Targeting CD38-mediated NAD+ metabolism might therefore represent a novel therapeutic approach to ameliorate chronic fibrosis.
“This is one of the first studies to find a relationship between CD38 and scleroderma, as well as the linking between inflammation and metabolism to skin and organ scarring,” says study author Johann Gudjonsson, M.D. Ph.D., a dermatologist at Michigan Medicine. “Now, we will look at designing and executing an early-stage ‘proof of principle’ clinical trial to assess the safety, tolerability, and efficacy of such innovative treatments in patients,” said Dr. Varga.

