Boosting NAD+ Improves Mitochondrial Health Following Cerebral Ischemia
Researchers from the University of Maryland and the Veterans Affairs Maryland Health Center recently found that NMN improves mitochondrial NAD+ metabolism and mitochondrial health via SIRT3 in ischemic mice.
Cerebral ischemia is defined by inadequate blood supply to neural tissue leading to an oxygen shortage. A person will lose consciousness after 10 seconds of interrupted blood flow to the brain, and other symptoms of cerebral ischemia include impairments in vision, body movement, and speaking abilities. Previous research has indicated treating mice with a molecule called nicotinamide mononucleotide (NMN) protects against ischemic brain damage and promotes cellular health by reducing fragmentation of the cell’s powerhouse, the mitochondria. However, the molecular mechanism by which this occurs remains unclear.
A group of scientists from The University of Maryland and the Veterans Affairs Maryland Health Center published a study in Experimental Neurology where they demonstrated that supplementing NMN to mice with cerebral ischemia improved mitochondrial health by reducing molecular tags called “acetylation” on proteins in the mitochondria. The administration of this molecule also reduced mitochondrial fission, a molecular process whereby the mitochondria split apart so that they are smaller in size, and reduced the quantity of reactive oxygen species–chemically reactive and physiologically damaging molecules–in cells.
NMN Restores NAD+ Levels and Improves Mitochondrial Structure
NMN boosts levels of a molecule called nicotinamide adenine dinucleotide (NAD+), an essential molecule supporting fundamental reactions in generating energy in the mitochondria. Researchers have proposed that NAD+ levels diminish following cerebral ischemia, affecting enzymes that depend on NAD+ to function. Sirtuins, such as sirtuin 3 (SIRT3), are NAD+ dependent enzymes that remove the molecular tags from mitochondrial proteins in a cellular process called “deacetylation.”
In the study, the scientists found that supplementing mice with NMN following cerebral ischemia boosted NAD+ levels and improved mitochondrial health in brain tissue via SIRT3 activation. NMN injections in mice following cerebral ischemia prevented both NAD+ depletion and increased mitochondrial protein acetylation that typically occurs after cerebral ischemia, demonstrating improved SIRT3 function.
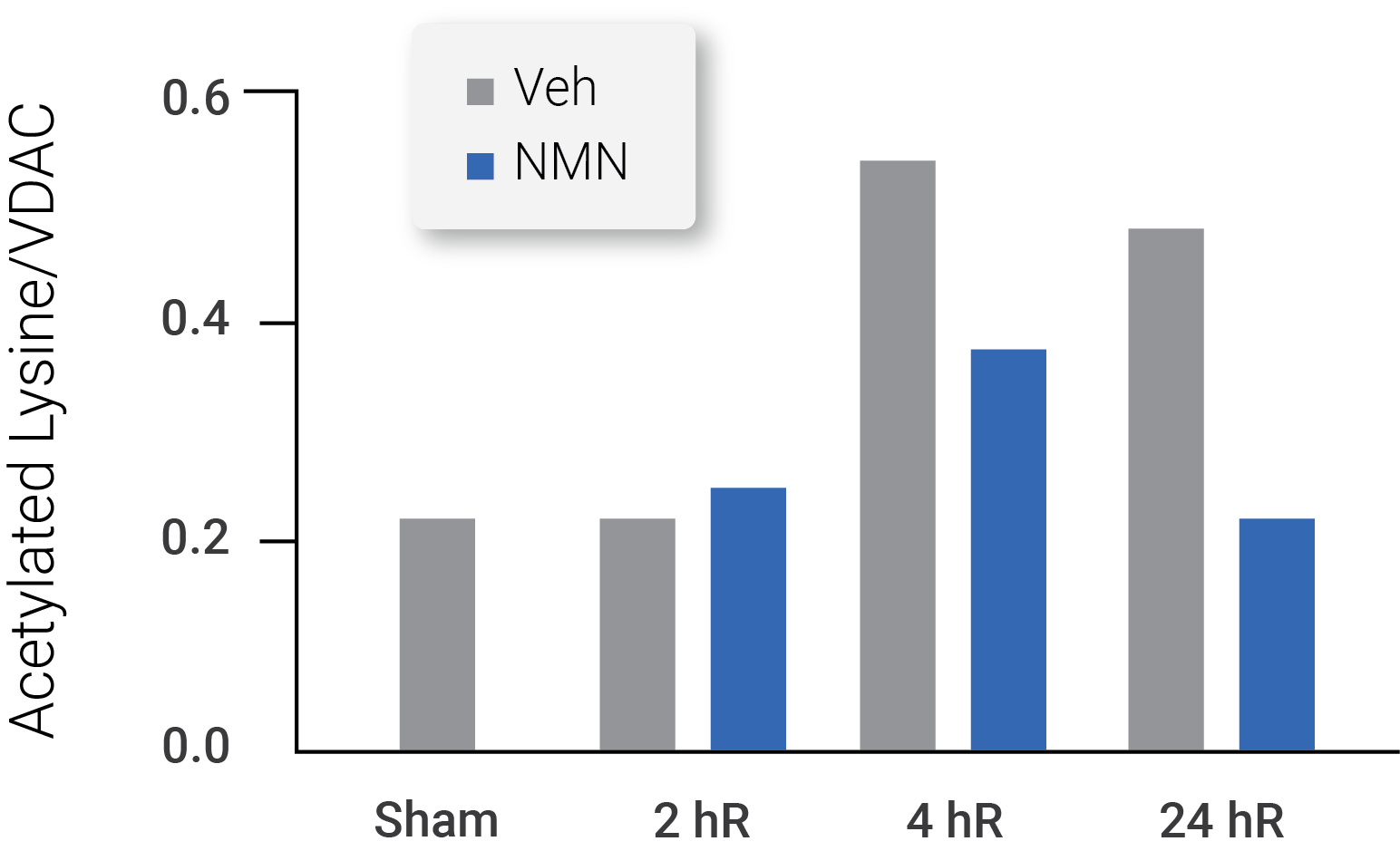
(Klimova et al., 2020 | Experimental Neurology) Mitochondrial protein acetylation analyses showed NMN treatment normalized acetylation at 24 hours following cerebral ischemia
The results of the study also showed NMN administration led to reductions in cerebral ischemia-induced mitochondrial fragmentation. Under normal circumstances, a balance exists in cells between mitochondrial fission and fusion. The process whereby mitochondria come apart to yield shorter mitochondria is referred to as “fission,” and the mitochondrial process of “fusion” denotes smaller mitochondria joining to form longer ones. In the study, mitochondria underwent fission following cerebral ischemia, and NMN supplementation reversed these effects.
Cerebral ischemia led to massive mitochondrial fission, with small, spherical mitochondria increasing from 30% to 50% of the mitochondria before and after cerebral ischemia, respectively. Moreover, longer, rod-shaped mitochondria represented 70% of the mitochondria before cerebral ischemia and fell to 50% after cerebral ischemia. Treatment with the molecule inhibited this post-ischemic mitochondrial fragmentation as the ischemic animals that received NMN had mitochondrial lengths that did not differ significantly from normal mice. The scientists found no statistically significant change in mitochondrial shape following cerebral ischemia in NMN treated mice.
To understand how NMN administration prevented mitochondrial fission, the scientists examined the concentration of pDrp1, a key protein in mitochondrial fission, following cerebral ischemia. The researchers found significantly increased levels of pDrp1 in brain mitochondria at 24 hours following cerebral ischemia, and NMN administration reversed these effects. Thus, the data indicated that administration of this molecule reduces mitochondrial fission by reducing levels of the fission protein, pDrp1.
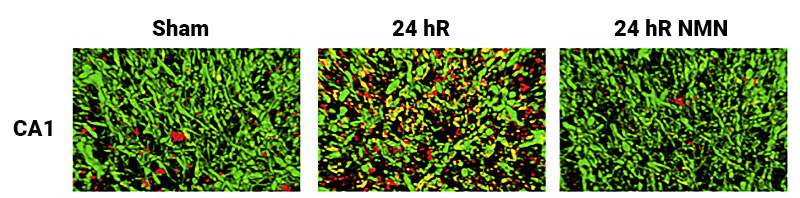
(Klimova et al., 2020 | Experimental Neurology) NMN administration reduced the concentration of the fission protein, pDrp1, in the outer membrane of the mitochondria in the brain. In the row of images, the pDrp1, which plays a key role in mitochondrial fission, is stained red. Normal mitochondria are presented in the left image with minimal pDrp1 staining. The middle image shows increased pDrp1 concentrations at 24 hours following cerebral ischemia. The image on the right shows a significant reduction in pDrp1 staining 24 hours after cerebral ischemia with NMN administration. The results indicate NMN administration reduces mitochondrial fission by reducing pDrp1 activity.
NMN treatment also decreased mitochondrial reactive oxygen species production, which can have damaging effects on cells. This indicated that this molecule could have a neuroprotective role in mice following cerebral ischemia. Following NMN administration, the researchers observed the reduced acetylation of an enzyme called superoxide dismutase 2, which breaks down reactive oxygen species. The reduced acetylation of superoxide dismutase 2 improved its activation and led to a reduction in reactive oxygen species buildup in mitochondria of the brain following cerebral ischemia.
“Our data provides a novel link between mitochondrial NAD+ metabolism, [reactive oxygen species] production, and mitochondrial fragmentation,” said the scientists in their publication. “Using NMN to target these mechanisms could represent a new therapeutic approach for neurobiological diseases.”

