Berberine Improves Spatial Memory in a Mouse Model of Alzheimer’s
Research published in Frontiers in Pharmacology shows that berberine reduces toxic protein accumulation in Alzheimer’s mice, providing new insights for future drug development in treating neurodegenerative diseases.
Highlights
· Berberine acts as an effective agent in relieving cognitive impairment in mice modeling Alzheimer’s disease.
· Levels of toxic proteins linked to Alzheimer’s disease called amyloid plaques and neurofibrillary tangles are improved with berberine treatment.
· The stress of a cell compartment called the endoplasmic reticulum, which is vital for responding to toxic proteins, is relieved by berberine in Alzheimer’s mice.
Although the exact root of neurodegenerative diseases like Alzheimer’s remains a bit of a conundrum, there’s a lot of evidence pointing to the role of toxic, improperly folded and shaped proteins called plaques and tangles that stress out and degrade nerve cells. Within all cells, a structure called the endoplasmic reticulum (ER) plays a role in properly assembling proteins and sensing when this process goes awry. However, when the ER becomes overloaded in detecting misshapen proteins in nerve cells, or what’s called the unfolded protein response, the ability to contain these virulent molecules spins out of control, leading to nerve cell death and deterioration of the nervous system.
Research published in Frontiers in Pharmacology shows that a natural compound called berberine can suppress ER stress to ameliorate the manifestation of plaques and tangles in a mouse model of Alzheimer’s disease. Wu and colleagues from Huazhong University of Science and Technology show that berberine halts the signaling of ER stress that triggers the formation of plaques and tangles, relieving the cognitive impairment observed in Alzheimer’s mice. These findings provide evidence for berberine and other compounds that improve ER stress may treat Alzheimer’s and other neurodegenerative diseases.
What Drives Alzheimer’s Disease?
The hallmarks of Alzheimer’s include amyloid plaques that occlude the outside of nerve cells and neurofibrillary tangles that clog up the inside. Amyloid plaques are deposits of aggregated proteins called amyloid beta (Aβ). To form neurofibrillary tangles, tau proteins become overly modified by a process called hyperphosphorylation, leading to clumping threads. Different signals trigger the production of amyloid plaques and hyperphosphorylated tau.
Recent studies have indicated several overlapping manifestations between these two, which also includes ER stress. Increasing studies have revealed that ER stress is observed in the postmortem brains of patients and animals with Alzheimer’s. But the precise mechanisms of how ER stress promotes the production of amyloid plaques and hyperphosphorylated tau are still not fully elucidated.
Relieving ER stress might affect the delay of progression and prevent the deterioration of Alzheimer’s disease. Meanwhile, a few studies reported amelioration of cognitive defects in Alzheimer’s mice by inhibiting the downstream sensors of ER stress. So, it is necessary to find a solution to signaling at the root of Alzheimer’s.
Berberine Ameliorates Cognitive Impairment in Alzheimer’s Mice
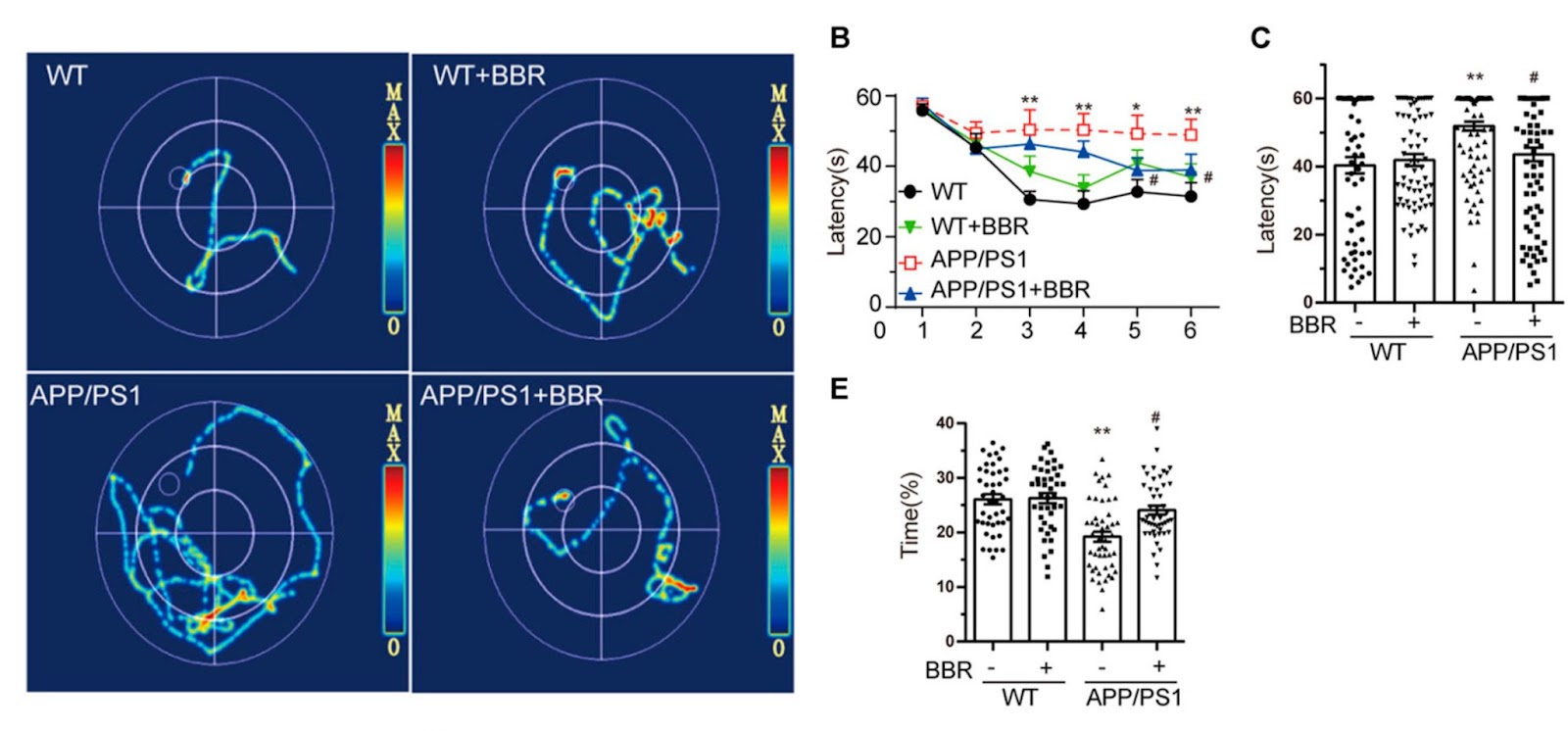
To determine whether berberine could ameliorate cognitive impairment in Alzheimer’s mice, Wu and colleagues employed the Morris Water Maze test to appraise the learning and memory abilities of Alzheimer’s mice fed normal food or food supplemented with berberine (260 mg/kg). In this test, mice, which are natural swimmers, are put into a cloudy circular pool surrounded by spatial cues and are tasked with finding a platform hidden in one of the quadrants. As the mice learn the spatial cues, the time it takes and the distances they need to swim to find the platform should decrease.
The results revealed that Alzheimer’s mice had trouble learning the spatial cues because the amount of time it took to solve the maze hardly improved with consecutive trials. On the other hand, berberine improved the time it took for Alzheimer’s mice to solve the maze to levels close to mice that did not model Alzheimer’s. The results also revealed that the Alzheimer’s mice spent a much shorter time staying in the target quadrant and berberine administration increased the time, indicating an improvement in spatial memory.
Berberine Reduces ER Stress and Toxic Proteins in Alzheimer’s Mice
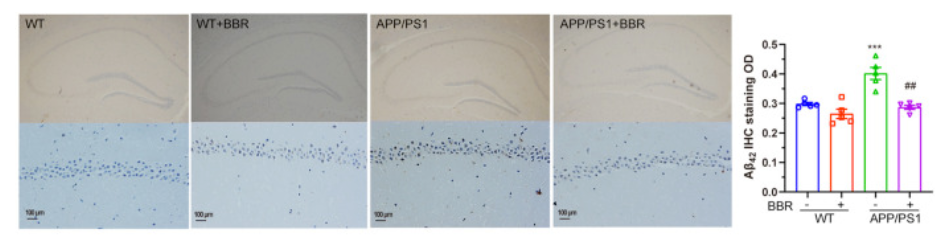
Consistent with the behavioral results, berberine treatment ameliorated the levels of both toxic proteins at the root of Alzheimer’s disease. What’s more, berberine improved the ER in Alzheimer’s mice. In non-Alzheimer’s mice, the ER is typically compact and elongated, but in Alzheimer’s mice, the ER becomes swollen. With berberine treatment, the shape of the ER returned to normal in the Alzheimer’s mice, which further supported that BBR treatment could alleviate ER stress. Taken together, these data indicated that berberine treatment could significantly alleviate ER stress in Alzheimer’s mice and attenuate two dominant disease-related changes of Alzheimer’s, which include the production and deposition of Aβ42 and tau hyperphosphorylation.
Wu and colleagues go on to show that ER stress could be the central signaling hub in the development of Alzheimer’s disease and that berberine treatment showed excellent protective effects to ER stress. Berberine was shown to be effective in inhibiting the hippocampal ER stress occurrence and downstream signaling pathways that lead to amyloid plaque formation.
In recent years, too many drugs, including ones that target amyloid plaques and the associated signaling pathways, have shown good therapeutic potential in early clinical trials, but turn out to be with largely disappointing results. One of the advantages of berberine in treating dementia is that it could travel through the blood-brain barrier, which is critical to affecting the nervous system. Berberine has already been shown to have low toxicity and gastrointestinal side effects after oral administration. Together with what is known about berberine, these findings point to the compound’s potential as a treatment for Alzheimer’s disease and potentially other neurodegenerative disorders based on ER stress and protein accumulation.

