Australian Study Shows NMN Delivers Stronger Bones
NMN promotes human bone formation and increases bone size in a mouse model for osteoporosis — age-related bone disease.
Highlights:
- NMN reduces senescent age-accelerating cells in human bone cell cultures.
- Treating human bone cells with NMN promotes bone formation.
- Osteoporotic mice injected with NMN display increased bone volume.
Though our bones become weak and prone to fracture with age, current therapies only modestly increase bone mineral density. This issue is largely due to the underlying causes of osteoporosis — reduced bone mass and density — being unclear. However, low NAD+ levels could be the culprit and NMN could be the answer.
In The Journals of Gerontology: Series A, Lu and colleagues from the University of Sydney in Australia now show that NMN alleviates human bone cell senescence and promotes bone healing in osteoporotic mice. These findings pave the way for NMN as a bonafide treatment for osteoporosis.
“This study would suggest NMN as an effective and feasible therapeutic candidate to prevent osteoporosis and enhance bone healing capacity in the osteoporotic older,” said the authors.
NMN Rejuvenates Bone-Forming Cells to Increase Bone Size
Like the rest of our organs, our bones are made of living cells. As such, old and damaged bone is constantly being replaced by new bone. However, with age, there are less bone-forming cells available, partially due to normal bone-forming cells becoming senescent cells. Senescent cells (which may drive the aging process in general) cannot form new bone, contributing to osteoporosis.
For this reason, Lu and colleagues examined the effect NMN on osteoporosis by studying human bone-forming cells. To induce senescence, the researchers exposed the bone-forming cells to a pro-inflammatory factor called TNF-⍺. While TNF-⍺ elevated senescence, treatment with NMN reduced this senescence by nearly 3-fold, suggesting NMN reduces senescent bone-forming cells.
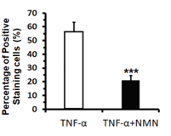
(Lu et al., 2022 | J. Gerontol) NMN Reduces Senescent Bone-Forming Cells. Senescence (Percentage of Positive Staining cell) induced by inflammatory TNF-⍺ was reduced by NMN (TNF-⍺+NMN).
Healthy bone-forming cells (osteoblast) form new bone tissue by transforming into mature bone cells (osteocytes). Lu and colleagues found that inducing senescence with TNF-⍺ reduced the abundance of mature bone cells. However, NMN increased the abundance of mature bone cells, suggesting that NMN promotes bone formation.
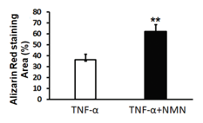
(Lu et al., 2022 | J. Gerontol) NMN Promotes Bone Formation. Decreased bone formation (Alizarin Red staining Area) induced by inflammatory TNF-⍺ was countered by NMN (TNF-⍺+NMN).
After determining that NMN reduces senescent bone-forming cells and promotes their differentiation into mature bone cells in a lab dish, Lu and colleagues tested if this could occur in living organisms. For this, they removed the ovaries of female mice and broke their femur bones, which led to lower bone volume, an indicator of osteoporosis.
To test the effect of NMN on osteoporosis, the researchers injected the osteoporotic mice with 400 mg/kg/day of NMN for 2 months. As a result, the bone volume of the osteoporotic mice was increased, suggesting that NMN partially reverses signs of osteoporosis. Combined with the human bone-forming cell data, this means that NMN may be able to treat osteoporosis by increasing bone formation.
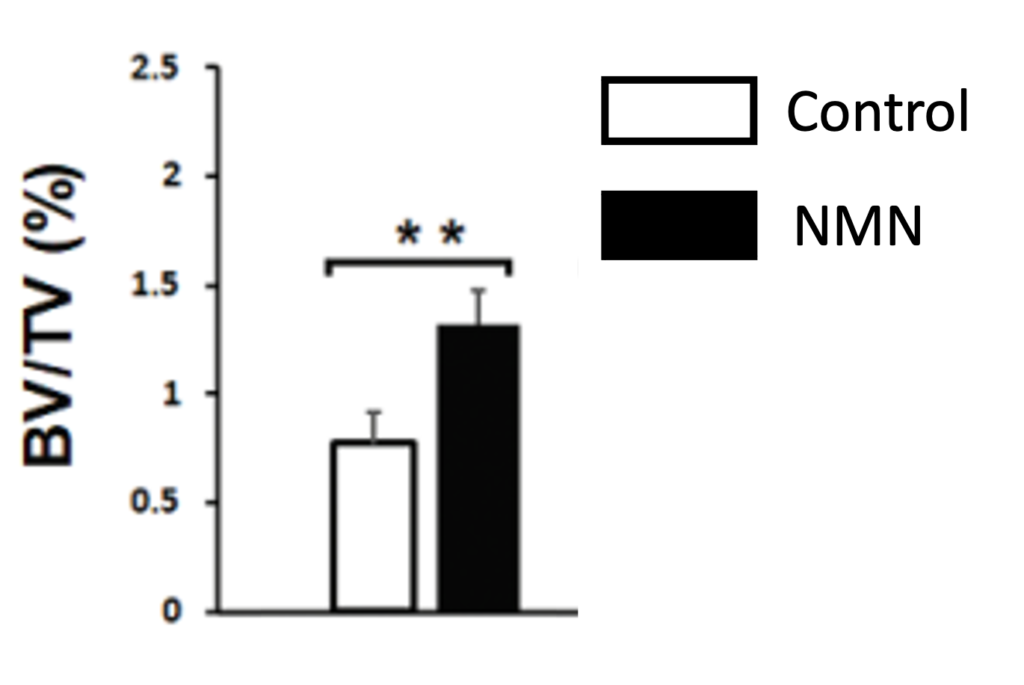
(Lu et al., 2022 | J. Gerontol) NMN Increase Bone Size. Untreated osteoporotic mice (Control) have lower bone volume (BV/TV) than osteroportoic mice treated with NMN (NMN).
NMN’s Bone Boosting Effects
Several previous animal studies suggest that NMN can boost bone formation. It seems to do so in several ways, including the rejuvenation of bone stem cells, which are essential for bone formation, for which NAD+ is indispensable. Bone stem cells differentiate into bone-forming cells, which Lu colleagues show are also rejuvenated by NMN.
These studies suggest that NMN may increase bone formation by boosting the health of several bone cells along the bone forming pathway. While there are no studies showing that NMN promotes bone formation in patients with osteoporosis, it’s possible that NMN can prevent bone loss with age.
Model: 12-month-old female C57BL/6 mice with ovaries removed to induce osteoporosis and femur bone fractured
Dosage: Intragastric injection of 400 mg/kg of NMN

