Are All Senescent Cells Bad?
While eliminating senescent cells is all the rage in the aging and longevity fields, there’s evidence for the beneficial roles of these growth-arrested cells.
Research on cell senescence has boomed in the last decade due to its pervasive implications. Senescent cells have gotten a pretty bad rap because these growth-arrested cells are known mostly for accumulating with age and causing various age-related diseases. And when certain populations of senescent cells get eliminated, researchers have been seen some remarkable changes, such as aging delay, age-related disease mitigation, and healthspan extension…at least in animals. While clinical trials have begun testing the safety and efficacy of senescent cell elimination in the context of age-related diseases, the direct clinical application of senescent cell clearance is currently not feasible.
Are all senescent cells bad?
When discovered in 1965, senescence was thought to serve as a means to suppress tumors. In essence, senescence isn’t inherently harmful, nor are all senescent cells bad. The molecules and compounds synthesized by senescent cells, or what’s called the senescence-associated secretory phenotype (SASP), play essential roles across the lifespan, including embryonic development, childbirth, and wound healing. For example, senescent cells are essential during embryonic development for fine-tuning the genesis of specific structures.
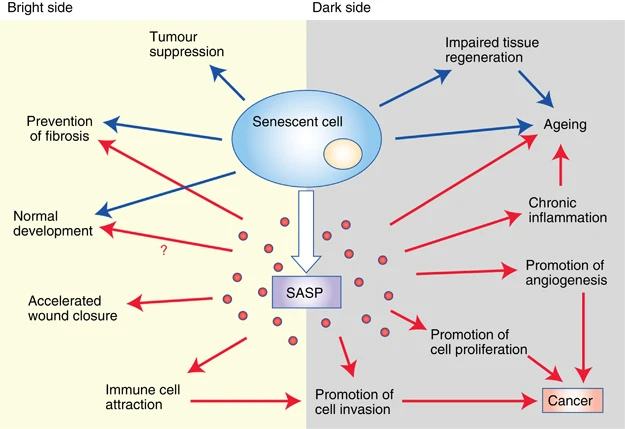
(Lecot et al., 2016 | British Journal of Cancer) Bright and dark sides of cellular senescence. On the bright side (left), senescence growth arrest prevents tumorigenesis and limits fibrosis. Certain features of normal embryonic development are promoted by senescent cells. Components of the SASP (senescence-associated secretory phenotype) accelerate wound closure and attract immune cells. On the dark side (right), the loss of proliferative potential that accompanies senescence impairs tissue regeneration and promotes aging. The SASP also contains factors that promote angiogenesis, cell proliferation, and cancer cell invasiveness. Furthermore, immune cells attracted by the SASP can disrupt the local microenvironment and promote tumor cell invasion. These activities result in cancer progression.
Senescent cells facilitate tumor suppression
While it is well known that the SASP can create a pro-tumorigenic microenvironment in several ways, senescent cells in some contexts recruit innate immune cells — the first line of defense against pathogenic and “foreign” sources — to kill nascent tumor cells. In particular, SASP factors recruit natural killer cells to eliminate malignant cells. In addition, SASP components attract immune cells, removing nearby damaged cells. Premalignant but senescent mouse liver cells secrete chemokines and cytokines that promote immune surveillance — the ability of the immune system to identify and destroy nascent tumors, thereby functioning as a primary defense against cancer. Impaired immune surveillance of these premalignant senescent liver cells fosters the development of certain liver cancers (hepatocellular carcinomas). Thus, senescence can also suppress tumor development through immune surveillance.
Atherosclerosis
In addition to suppressing tumor proliferation, senescence may play a similar role in other types of detrimental cells. For example, increased cell cycle levels inhibiting gene activity have been associated with reduced susceptibility to atherosclerotic vascular disease. Along these lines, an emerging view is that gene activation of cell cycle inhibitors in macrophages limits atherosclerotic plaque growth. But while gene activation of cell cycle inhibitors appears to prevent atherosclerotic plaque formation, it also seems that the SASP contributes to generating atherosclerotic plaques (atherogenesis). These results suggest a complex model in which the anti-proliferative component of senescence (e.g., CDKN2A activation) is beneficial while other aspects of senescence (e.g., SASP production) are detrimental by contributing to atherogenesis.
Senescent cells fine-tune structures during development
Cells with features of senescence have been identified in several transient anatomical structures in the developing embryo and appear to play a role in shaping organogenesis. Such cells are seemingly senescent — they hardly replicate — yet don’t share all of the same features as age- and disease-related senescent cells. For example, these developmental senescence-like cells are not associated with DNA damage, do not depend on molecules linked to proliferative arrest, and do not secrete the typical range of SASP compounds. Instead, embryonic senescence is programmed into our DNA to be executed during development.
Senescent cells participate in tissue repair and wound healing
In addition to stopping the proliferation of premalignant cells, some data suggest that senescence has other beneficial effects. In response to acute liver injury, specific liver cells, called hepatic stellate cells, are induced to replicate and produce web-like structures on the outside of cells called the extracellular matrix (ECM) to repair the damage but then undergo senescence. In mice lacking senescence effectors, hepatic stellate cells proliferate and produce excessive ECM, leading to tissue scarring (fibrosis). Thus, liver injury induces a senescence response, limiting liver fibrosis; this effect is critically dependent on enzymes that degrade ECM that comprise part of the SASP.
Although the limitation of liver fibrosis is a critical consequence of senescence, other models of tissue injury reveal additional roles for senescent cells in response to injury. Indeed, senescent cells and the SASP are crucial for optimal wound healing. In response to cutaneous wounds, fibroblasts and endothelial cells undergo senescence. This senescence accelerates wound closure by inducing myofibroblast differentiation through the secretion of a growth factor called PDGD-AA that influences immune cells, amongst others. When researchers have selectively eliminated senescent cells using genetically altered mice, they observe delays in wound closure. Similarly, topical treatment of senescence-free wounds with PDGF-AA rescues the delayed wound closure.
Senescence facilitate immune and anti-viral responses
Senescence plays complex roles in both cellular and innate immunity. In old organisms, certain immune cells called T cells — which attack pathogens and activate other immune cells — have high levels of senescent markers and a subset of SASP factors. This T cell senescence can be triggered by aging or chronic HIV infection and has been suggested to lead to a loss of diversity in the T cell repertoire and drive immune aging.
In contrast, given that certain viruses depend on host cell proliferation for viral replication, it has also been postulated that cellular senescence may have evolved as a host anti-viral defense. Viral infection can induce cellular senescence directly by causing cell fusion or DNA damage, and perhaps indirectly via prolonged cytokine signaling with the induction of nearby “paracrine” senescence. The replication of certain viruses has been demonstrated to be impaired in senescent cells, and senescent cells can recruit innate immune cells that might prevent the further spreading of a viral infection. Therefore, senescence has been argued to provide a natural barrier to certain types of infection by limiting host cell proliferation and as a means to activate innate immunity but may compromise cellular immunity with aging or chronic infection.
Thus, there is a beneficial role for senescence and the SASP in development and tissue repair — a critical ‘bright side’ of senescence that extends beyond tumor suppression.

(He & Sharpless 2017 | Cell) The Beneficial Roles of Cellular Senescence. Senescence (1) affords tumor suppression and augments local anti-tumor immunity, (2) limits the size of atherosclerotic plaques, thereby reducing anatomic obstruction, (3) may be required for certain aspects of fetal development, and (4) contributes to wound healing and host immunity.
Can specific senescent cells be targeted for elimination?
Another complication is that differences in cell type, origin, location, and disease can significantly affect senescent cells. Senescent cells are variegated in form and function, as this growth-arrested state has been observed in many different cells types from other tissues caused by different kinds of stressors. Amid this evolving appreciation of senescent cell diversity, there are likely yet undefined populations and subpopulations of senescent cells that may have distinct characteristics. For example, senescent cells probably possess unique spatial and temporal dynamics as well as fulfill distinct physiologic and disease-causing roles in a context-dependent manner.
Since senescent cells are very diversified, current strategies that target a general population of senescent cells are less effective. Therefore, it will be vital to carefully evaluate the type of cell population and the best model to use before starting any possible treatment. Ideally, as our understanding of senescence variability expands, it will be increasingly possible to use rational design strategies to target and eliminate only the most detrimental senescent cell subpopulations.
Is it safe to kill off senescent cells?
A further challenge limiting the use of targeted senescent cell clearance is the safety of the potential treatments. Senolytics under consideration could potentially have side effects in non-senescent cells and also interfere with any of the beneficial aspects of senescent cells. As previously mentioned, senescent cells participate in tissue repair and regeneration and tumor suppression, but the effects of targeted senescent cell clearance on these beneficial aspects remain largely unexplored.
So, safety assurance must be a primary consideration for any strategies that translate senescent cell clearance into clinical treatment. Drugs with improved target recognition must be developed, in conjunction with further investigations into drug activation factors, such as specific enzymes.
Ultimately, killing senescent cells risks losing their beneficial effects. Thus, although the early preclinical results are promising, senescence-targeted therapies are currently in their infancy. Future studies will determine the best use of therapies to exploit the context-dependent nature of senescent cells.

