Scientists’ Find New Angle for Alzheimer’s Treatment
The study provides a proof of concept for gene activation therapy in attenuating Alzheimer’s Disease progression including cognitive decline.
Highlights
- An enzyme that helps protect chromosome ends called TERT is downregulated in Alzheimer’s disease.
- TERT activation alleviates toxicity and enhances brain connectivity in an Alzheimer’s disease mouse model.
- In human cells with Alzheimer’s, TERT activation alleviates toxicity in neurons and increases gene activity related to learning and memory impairment.
Alzheimer’s disease is the most common type of dementia and is characterized by the accumulation of toxic protein aggregates in the brain, leading to progressive neuronal loss (neurodegeneration) and a decline in learning, memory, and other cognitive functions. What’s more, researchers estimate the number of people with dementia will nearly triple to more than 152 million by 2050. These insights have spurred efforts to identify therapies designed to reduce toxic protein aggregate accumulation and preserve the survival and function of neurons.
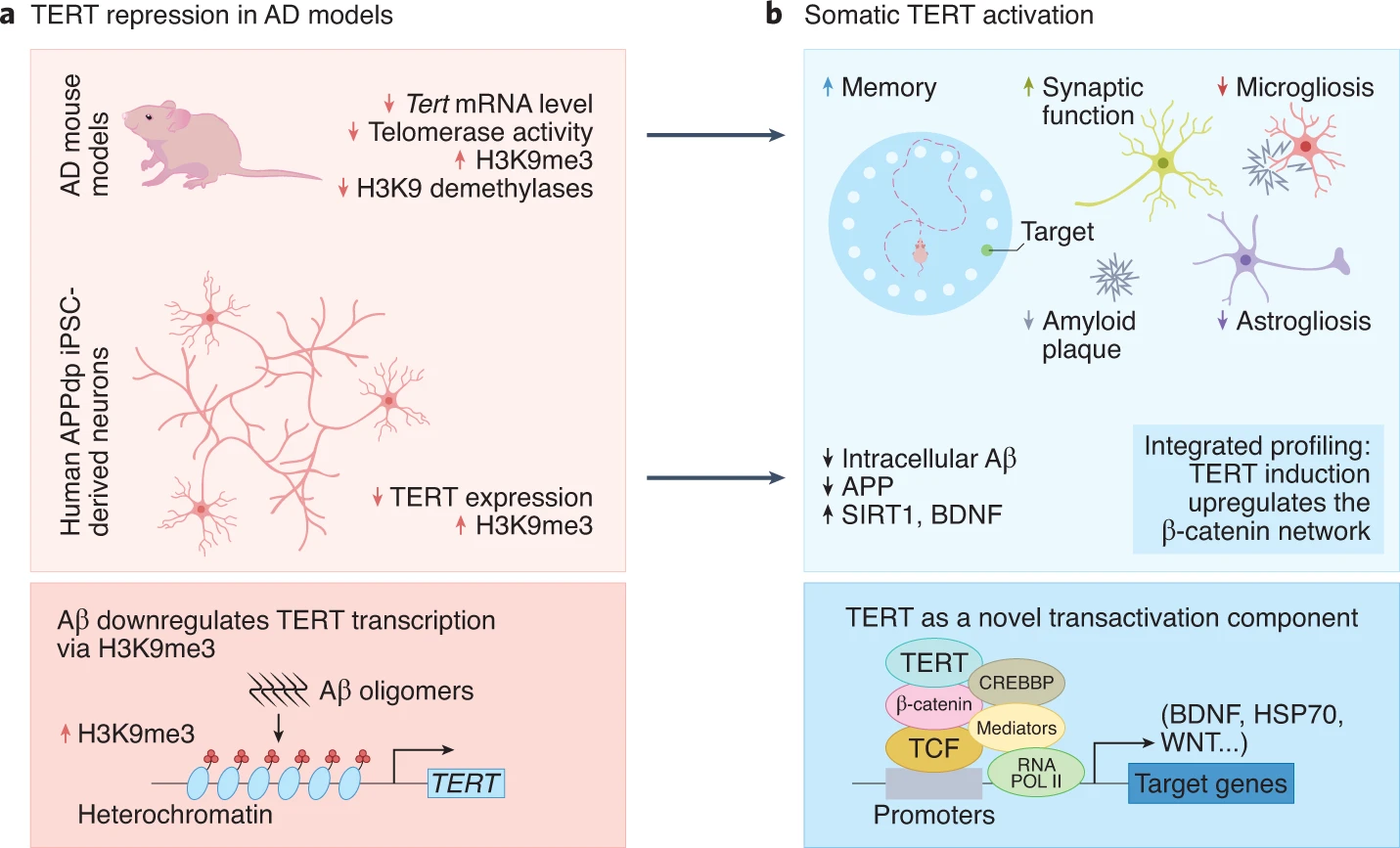
A research team from The University of Texas MD Anderson Cancer Center demonstrate that activation of a protein that maintains the protective caps on chromosome ends prevents Alzheimer’s disease progression. Published in Nature Aging, this study shows that increasing levels of the TERT (telomerase reverse transcriptase) protein in mouse and human neurons afflicted with Alzheimer’s disease reduces toxic protein aggregate accumulation while preserving neuron connectivity and cognitive function. These findings from Shim and colleagues provide genetic proof of concept for TERT gene activation therapy in attenuating Alzheimer’s disease progression including cognitive decline.
The Therapeutic Potential of Telomere Protection in Neurodegeneration
Telomerase is a protein and DNA complex responsible for maintaining the length of telomeres — the protective caps and chromosome ends — and genome integrity. Mice engineered to experience telomere dysfunction exhibit premature aging characteristics including neurodegeneration, and aging human brains with Alzheimer’s disease also show signs of DNA damage-related stress in neurons. With a critical role in aging and age-related diseases and their activation, telomerase activation has been proposed as a potential therapeutic for human aging and age-related degenerative diseases.
TERT Levels and Alzheimer’s Disease Are Closely Connected
Emerging evidence has suggested that TERT is a potent protector from brain aging and age-associated diseases. In this study, Shim and colleagues explored whether and how modest changes in TERT levels might also influence neurons of the normal mouse brain and regulate disease-driving processes driving neurodegenerative diseases like Alzheimer’s disease.
The MD Anderson Cancer Center researchers show that inactivation of the TERT gene in the mouse brain leads to decreases in the levels of critical hormones that promote neuron health and survival while increasing toxic protein aggregates. Also, in mouse and human Alzheimer’s neurons, the TERT locus appears to get shut down, implicating TERT repression in neurodegeneration induced by toxic protein accumulation.
TERT Activation Alleviates Alzheimer’s Disease
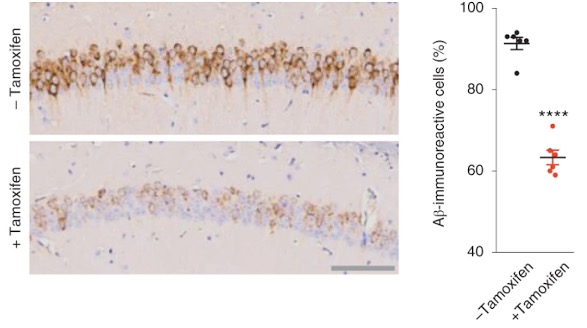
To test the impact of sustained TERT expression on neurodegeneration, Alzheimer’s disease mouse models were engineered to maintain normal levels of TERT in adult neurons. Shim and colleagues established that increases in TERT levels resulted in a marked reduction of toxic protein aggregate levels in neurons in the brains of mice and in cultured human neurons modeling Alzheimer’s disease.
The loss and dysfunction of the sites of nerve cell connections (synapses) are major correlates of cognitive decline in Alzheimer’s disease. In this study, TERT induction was linked to increases in the density of dendritic spines — structures for receiving nerve signal transmission — in the brains of Alzheimer’s disease mice, suggesting improved neuronal connectivity. Specifically, the enhanced dendritic spine density was observed in the outermost layer of the brain called the cerebral cortex, which is where complex, high-order functions (e.g., attention, perception, awareness, thought, language, and consciousness) are executed. Shim and colleagues conclude that elevated TERT levels in neurons activate synaptic signaling cascades and reduce spine shrinkage and synaptic loss in neurons of the mouse Alzheimer’s brain.

Shim and colleagues next investigated whether TERT activation could ameliorate the learning deficits of Alzheimer’s disease models. To do so, they used a test called the Barnes maze, which consists of a circular table with holes around the circumference, placed in a room with visual cues in the periphery. Most of these holes lead to an open drop to the floor, but a single hole leads to a dark box in which the animal can hide, also known as the “drop-box.” After a few trials, rodents typically remember which hole contains the drop-box and quickly proceed in a direct path toward the hole. Investigators can measure the amount of time to find the correct hole, the number of incorrect holes explored, and the length of the exploratory path.
As expected Alzheimer’s disease mice showed impaired acquisition of spatial learning at old age on the Barnes maze. But the Alzheimer’s disease mice with activated TERT achieved significant prevention of learning and memory dysfunction, as indicated by a reduction in latencies to enter the escape hole. These findings, which align with the above cellular and molecular data, indicate that TERT activation attenuates the age-associated learning impairment of Alzheimer’s disease mice.
TERT Enhances Learning Pathway Networks In Human Neurons
These observations in mouse models of Alzheimer’s diseases prompted Shim and colleagues to assess the biological impact of TERT induction in a well-established stem cell (iPSCs) model generated from a patient with Alzheimer’s disease. Mirroring the mouse findings, the TERT gene was shut down in the human neurons and TERT induction resulted in a reduction of intracellular Aβ accumulation. Furthermore, TERT induction not only decreased toxic protein levels but also triggered activation of genes known to be crucial in reducing Aβ processing and toxicity and improving synaptic plasticity and memory formation in the adult brain. Going one step further, intersecting the gene and pathway activity profiles of mouse and human neurons from Alzheimer’s disease models revealed that TERT activation enriched learning processes in these cells.
Counteracting Alzheimer’s Disease Through TERT Activation
These findings suggest that TERT regulates critical disease-associated pathways in Alzheimer’s disease and provide a deeper understanding of how TERT contributes to neuronal health in the setting of neurodegeneration and cognitive deficits in both mouse and human models of Alzheimer’s disease. This study justifies the testing of various somatic TERT therapies, such as small-molecule activators or inactivation inhibitors of TERT gene activation as potential therapeutic options for patients with Alzheimer’s disease.

