A Sneak Peek of Sinclair’s MetroBiotech Lab and New NMN Clinical Trials
A first look inside the labs of Metro Biotech, a company co-founded by David Sinclair that is testing the effects of NMN in three exciting new clinical trials.
Highlights:
- Metro Biotech just released a video showing their lab and describing some of their projects, including potentially making new NAD+ precursors into drugs.
- The video mentions three Phase 2 clinical trials for MIB-626 NMN that are currently recruiting.
- Metro Biotech believes NAD+ precursor treatment may potentially treat over 50 diseases by targeting mitochondria.
Metro International Biotech is a pharmaceutical company founded by Harvard professor Dr. David Sinclair and Washington University professor Dr. Rajendra Apte. The company focuses on NAD+ precursors, particularly NMN. In a recent tweet, Sinclair said,
“Metrobiotech has been keeping their head down for a decade, developing new classes of medicines that modulate NAD+ to treat rare and common diseases. Here’s a first look inside.”
In the video, shared in Sinclair’s tweet, the inside of the Metro Biotech lab, located in Massachusetts, can be seen. Here, researchers have discovered novel NAD+ precursors that have never been previously made into drugs. According to the researchers in the video, they plan to use these NAD+ precursors to treat diseases. They are also working on ways to inhibit NAD+ synthesis to treat cancer.

Metro Biotech Clinical Trials
In the video, Metro Biotech’s CEO David J. Livingston mentions that they are conducting three FDA-approved Phase 2 clinical trials:
The effect of NMN on healthy individuals: This trial will test the effect of 2 g of MIB-626 NMN on 120 highly physically fit, regularly exercising men and women between the ages of 19 and 40. The primary objective will be to determine if NMN improves cardiorespiratory fitness, as measured by VO2 max, in response to NMN + normal activity, or NMN + “progressive high-intensity exercise training.” Secondary outcome measures include:
- Endurance
- Muscle performance
- Attention and reaction time
- Working memory
- Sleep
- Insulin sensitivity
- Blood lipids
The effect of NMN on Alzheimer’s disease: This trial will test the effect of 2 g of MIB-626 NMN on Alzheimer’s patients. The primary objective will be to determine if NMN can cross the blood barrier by measuring its presence in the cerebral spinal fluid (CSF). Other outcome measures include:
- NAD+ brain concentrations
- Pathological amyloid-beta concentrations
- Neurodegeneration
- Cognition
The effect of NMN on diabetes kidney diseases (DKD): This trial will test the effect of 2 g of MIB-626 NMN on patients with DKD — a common complication with type 1 and type 2 diabetes that damages the kidney. The primary objective will be to determine if NMN can counteract kidney damage and dysfunction. Secondary outcome measures include:
- Muscle endurance
- Walking performance
- Inflammation
- Blood glucose concentrations
Treating 50 Rare Diseases by Targeting Mitochondria
In the video, Metro Biotech’s CEO mentions using NAD+ to treat rare diseases caused by dysfunctional mitochondria. A page on Metro Biotech’s website expands on Livingston’s comment, explaining that over 50 diseases are considered rare mitochondrial diseases. These diseases come with serious side effects that affect many organ systems, including the brain, heart, and muscle.
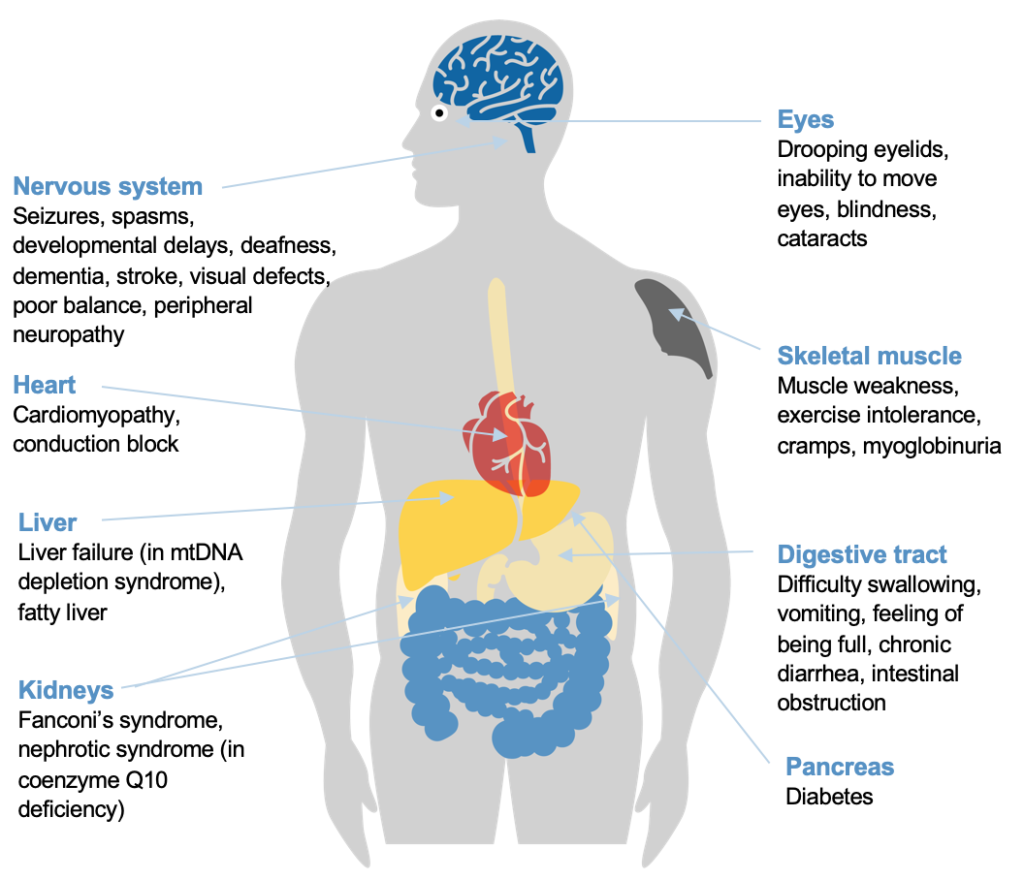
Since adequate NAD+ levels are intimately connected to mitochondrial health, the scientists at Metro Biotech hope to develop treatments for rare mitochondrial diseases with NAD+ precursors.
Prospects for MIB-626 Drug
If Metro Biotech’s clinical trials are a success, we may soon be seeing MIB-626 NMN tested in Phase 3 clinical trials. If those go well, it’s possible that MIB-626 could receive the FDA’s approval as a drug for the treatment of diseases like Alzheimer’s and DKD. It could even potentially be approved for improving heart function if the trial in healthy individuals goes well. If approved for multiple age-related indications, MIB-626 could be the first true anti-aging drug in history.

