A Drug that Removes Senescent Cells Extends Life, New Japanese Study Reveals
The diabetes drug canagliflozin removes age-promoting senescent cells and extends the lifespan of mice.
Highlights
- Canagliflozin reduces senescence in the fat tissue of obese mice.
- Canagliflozin protects against heart disease in a mouse model for atherosclerosis.
- Canagliflozin prolongs the life of prematurely aged mice and improves the strength of naturally aged mice.
The latest research suggests that senescent cells accumulate in our body because our immune system weakens with age. Without removal, senescent cells promote chronic inflammation and contribute to the development of multiple diseases, including cardiovascular disease.
A weak immune system could potentially be made up for with compounds that remove senescent cells, called senolytics. While a host of senolytics have been identified, including the naturally occurring fisetin and quercetin, FDA-approved drugs can also be repurposed as senolytics. With that being said, Japanese researchers have recently discovered that canagliflozin (cana), an FDA-approved diabetes drug, exhibits senolytic properties.
Canagliflozin Reduces Senescence
The Japanese researchers, Katsuumi and colleagues tested the effects of cana on mice fed a high-fat diet. Feeding mice a high-fat diet induces obesity, increasing the amount of fat carried by the mice. In examining the fat of the obese mice, the researchers found an increase in senescent cells and indicators of inflammation. However, if the obese mice were fed cana for only one week, both the senescent cells and inflammation were reduced.
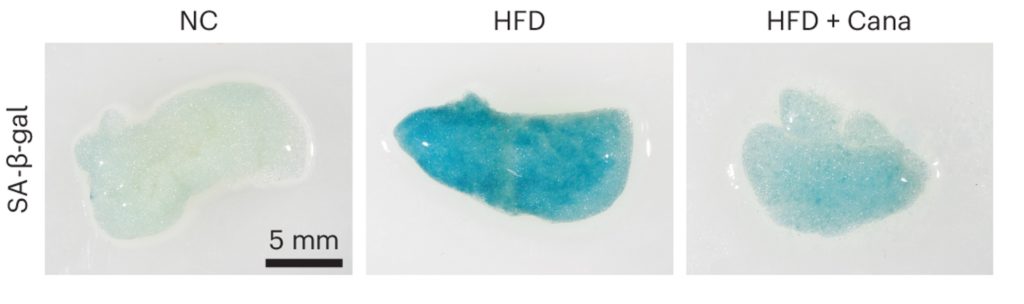
To determine how cana reduces the accumulation of senescent cells, the researchers measured which molecules are activated in response to cana treatment. They found that the longevity-associated enzyme AMPK (AMP-activated protein kinase) was activated in response to cana. AMPK plays a crucial role in energy metabolism and is naturally activated during prolonged fasting, whereby glucose levels are low. AMPK activation delays aging and prolongs the lifespan of several species, in part by stimulating autophagy, our cellular waste disposal and recycling system.
Katsuumi and colleagues found that cana-induced AMPK activation directly targets senescent cells that are resistant to removal by the immune system. These stubborn immune system-evading senescent cells (expressing PD-L1–PD-1) are more prone to accumulation and contribute more strongly to biological aging than other senescent cells. Importantly, cana was shown to increase the level of immune cells that destroy these senescent cells via activation of AMPK.
Canagliflozin Protects Against Heart Disease
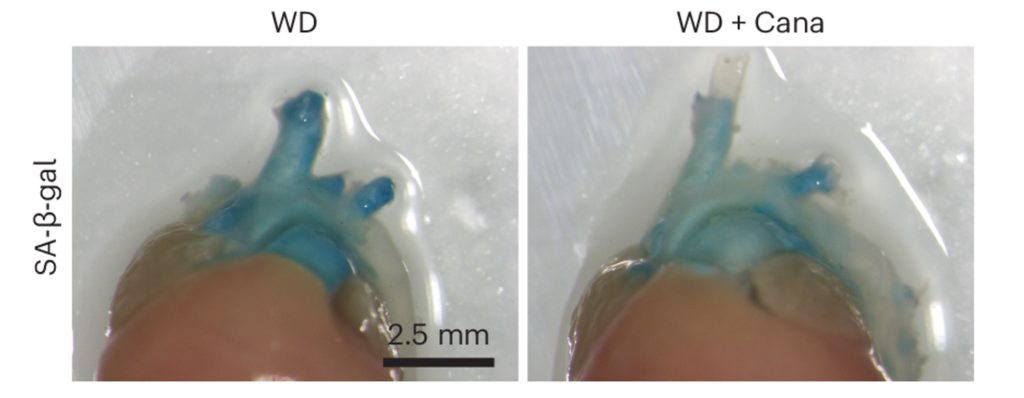
Atherosclerosis is a form of heart disease characterized by the buildup of fatty plaques in the arteries that can lead to heart attacks and strokes. Atherosclerosis is the leading cause of death worldwide. In a mouse model for atherosclerosis, Katsuumi and colleagues found that cana treatment reduced artery plaque size while reducing senescent cell accumulation and inflammation in the aorta. These results corroborate with a study showing that cana reduces the risk of cardiovascular death in type 2 diabetes patients.
Canagliflozin Prolongs Life and Improves Strength
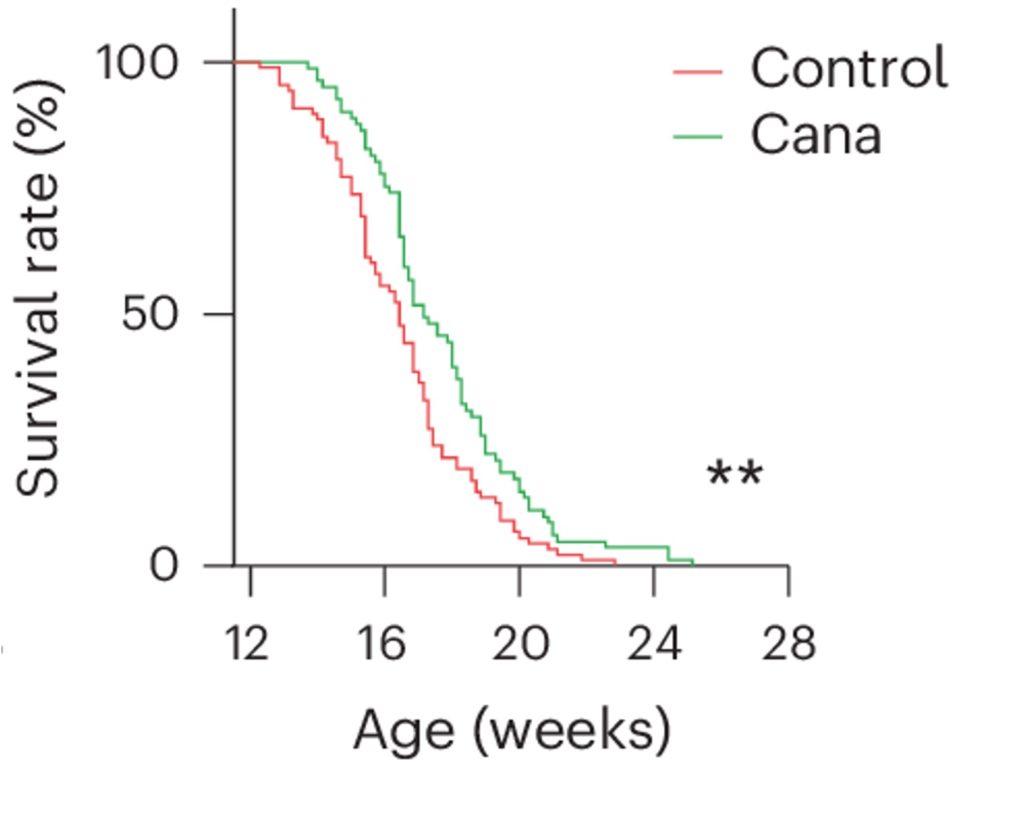
Hutchinson–Gilford progeria syndrome is a disease that causes premature aging. To determine if it could delay premature aging, Katsuumi and colleagues treated a mouse model for Hutchinson–Gilford progeria syndrome with cana. For both male and female mice, cana led to an increase in lifespan, suggesting that cana prolongs life. However, cana does not appear to prolong the lifespan of naturally aged mice. Still, in the case of naturally aged mice, it was shown that cana increases grip strength and improves balance.
Study Flaw
Cana alleviates diabetes by increasing the urinary excretion of glucose by inhibiting a protein called SGLT2 (sodium-glucose cotransporter-2) in the kidney. Glucose carries calories, so excreting it is similar to reducing calorie intake. Since reducing calorie intake has been shown to reduce the accumulation of senescent cells, Katsuumi and colleagues tested whether cana reduces the accumulation of senescent cells by lowering blood glucose levels.
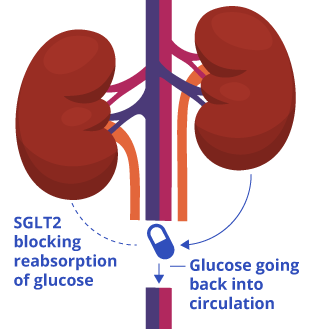
The problem is that the researchers lowered blood glucose levels by administering insulin to the mice. Insulin does not lead to urinary glucose excretion but causes glucose to go into cells where it is converted into cellular energy. Based on this insulin experiment, the authors concluded that cana does not remove senescent cells by lowering blood glucose. However, this is not accurate, as glucose leaving the body is not the same as glucose going into cells.
AMPK is activated in the absence of glucose within cells, so if glucose is excreted from the bloodstream via cana, AMPK may be activated. In contrast, AMPK may not be activated if glucose enters cells in response to insulin. Therefore, cana could potentially activate AMPK by reducing glucose levels, which the authors of the study do not explicitly point out.
Can Limiting Sugar Intake Delay Aging by Reducing Senescence?
While prolonged fasting may be out of the question for some individuals, it is still possible that taking pharmaceutical drugs like cana is unnecessary for activating AMPK and reducing the accumulation of senescent cells. Limiting sugar intake could reap the same benefits.
The ketogenic diet, which drastically limits carbohydrate and sugar intake, has been shown to prolong the lifespan of mice. At least one study has shown that a low-carbohydrate diet also prolongs lifespan, but these results were not significant. The ketogenic diet is known to activate AMPK in the brain, liver, and muscle. Ketosis-induced AMPK activation leads to the inhibition of mTOR, one of the most potent interventions for prolonging the lifespan of mice.
A low-carbohydrate diet (15% of calories from carbs) has also been shown to activate AMPK by lowering blood glucose levels in diabetic mice. However, considering that most Americans get about 50% of their calories from carbs, it may be difficult for most individuals to strictly follow a low-carb diet. More studies will needed to determine the threshold of carbohydrate intake needed to activate AMPK and potentially reap the benefits of cana.

