Unlock Longevity: Study Reveals Gene Therapy Boosts Mouse Life
Researchers at Rejuvenate Bio — a San Diego-based biotech company — reveal that a gene therapy technique that rejuvenates cells also dramatically prolongs the remaining lifespan of aged mice.
Highlights
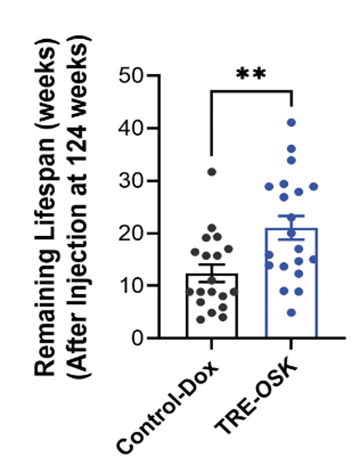
- The gene therapy extended the remaining lifespan by 109% for 124-week-old mice (equivalent to ~77 years for humans).
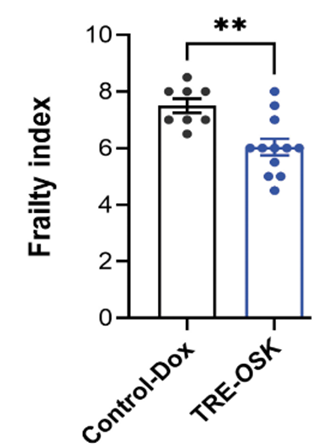
- This technique also reduced age-related frailty, where muscles become weak and bones become delicate.
- Aged mice and human skin cells showed age reversal as measured with an analysis of DNA molecular tagging patterns following gene therapy.
In a Cellular Reprogramming publication, Davidsohn and colleagues from the San Diego-based biotech company Rejuvenate Bio show that a gene therapy technique called partial cellular reprogramming dramatically extended the remaining lifespan of normal, aged mice by 109%. Moreover, this gene therapy technique alleviated age-related frailty, a condition where muscles weaken and bones become brittle. The aged mice as well as human skin cells also showed age reversal as indicated by an analysis of DNA molecular tagging patterns — epigenetic age — following the gene therapy.
This is a noteworthy leap since a previous study showing partial cellular reprogramming extended lifespan had been done with mice that have an accelerated aging condition — progeria. Since most people who could potentially benefit from future gene therapy developments do not have an aging condition like progeria, these findings with healthy mice have a higher probability of application to humans.
The Partial Cellular Reprogramming Technique Explained
Partial cellular reprogramming is a technique used to make cells younger through the transient delivery of genetic material so that cells produce proteins called Yamanaka factors. Partial cellular reprogramming is derived from another method called cellular reprogramming. Cellular reprogramming exposes cells to the Yamanaka factors for longer periods of time. Exposing cells for longer periods has been shown to revert aged cells to stem cells.
When cells are reprogrammed into stem cells, they reach a state called pluripotency — where they can grow into any other type of cell in the body. However, once cells reach this state, they lose their niche function within the organ from which they come. This scenario is undesirable since we need cells in the body to maintain their niche function as a specific cell type, like a liver cell (hepatocyte), to perform crucial tasks in certain organs, such as the liver.
Exposing cells to the Yamanaka factors for shorter durations has helped researchers revitalize cells without turning them into stem cells where they lose their specific functions within certain organs. Hence, exposing cells to Yamanaka factors for shorter periods is referred to as partial cellular reprogramming, as opposed to cellular reprogramming, since cells are rejuvenated and not reverted to stem cells.
Gene Therapy Injections Dramatically Extend Remaining Lifespan, Alleviate Frailty, and Reverse Aging
For their experiment, Davidsohn and colleagues used normal, as opposed to genetically altered, 124-week-old mice — equivalent to about 77 years old in humans. The researchers utilized aged mice since humans who might use anti-aging gene therapies in the future would likely also be older.
Remarkably, injecting the aged mice with non-infectious viruses containing the OSK genes and then inducing OSK activation extended the aged mice’s lifespan by 109%. For comparison, mice that did not undergo gene therapy lived an additional 8.86 weeks past their initial age of 124 weeks, while gene therapy-treated mice survived 18.5 weeks longer. The added 9.64 weeks from gene therapy would translate to about an 18.9-year lifespan extension for humans.
Not only that but gene therapy-treated mice showed markedly reduced frailty as measured with a frailty index. This analysis factored in things like sensory and motor tests as well as physical condition. Since the gene therapy alleviated frailty, this would improve the mice’s quality of life (healthspan) throughout their prolonged lifespan.
If that is not enough, treatments with the gene therapy reversed aging in the liver and heart as assessed with DNA molecular tagging patterns — epigenetic age. In other words, the difference between these organs’ predicted ages based on epigenetic age measurements (biological age) was lower than their ages in weeks (chronological age).
To get a better handle on whether this therapy could be applied to humans, Davidsohn and colleagues isolated skin cells from the scalp of a 65-year-old man and applied the gene therapy. Upon doing so, the researchers found cellular age reversal as shown with epigenetic age analysis. These results support that the lifespan-extending partial cellular reprogramming gene therapy can be applied to human cells as well. Further testing will be necessary to find whether this gene therapy works for living humans.
Applying Partial Cellular Reprogramming to Human Lifespan Extension
The promising findings that partial cellular reprogramming gene therapy drastically extends the remaining lifespan of aged mice begs the question of when humans will reap the benefits. Along those lines, since gene therapies are often risky due to increasing the risk of cancer as well as triggering detrimental immune responses, we may not even need gene therapy for cell and tissue rejuvenation. In fact, Harvard’s David Sinclair identified six cocktails combining known compounds that reprogram human cells to a younger state. Although testing these compounds’ effects in animals and ultimately humans is still required, the possibility of rejuvenating cells and tissues with pharmaceuticals could be an alternative to gene therapy.
In the same regard, Stanford genetics professor and entrepreneur Dr. Ronjon Nag is attempting to develop a vaccine against aging in the next seven years. One of the anti-aging strategies he is targeting by combining already FDA-approved drugs for the vaccine is cellular rejuvenation through partial cellular reprogramming. Based on Dr. Nag’s artificial intelligence (AI)-driven identification of compounds (which he will not specify) that rejuvenate cells through this method, it seems likely that compounds already exist with this capacity. In that sense, we may one day be able to skip the riskiness of gene therapy altogether while reaping the benefits of partial cellular reprogramming.
If researchers can develop a cocktail of compounds for partial cellular reprogramming within the next seven years, the Food and Drug Administration (FDA) would still need to approve it before human use. Since the FDA approval process for a new vaccine usually takes between five to ten years, adding Dr. Nag’s targeted seven-year timeframe for developing the vaccine means we could see it as early as 12 to 17 years from now. Alternatively, if the development of partial cellular reprogramming through gene therapy works, the FDA could theoretically approve it within the next 12 to 15 years.
Model: 124-week-old male C57BL6/J mice
Dosage: AAV9.TRE3-OSK-SV40pA (1.556 E13 vg/mL) and AAV9-hEf1a-rtTA4-Sv40pA (1.88 E13 vg/mL) viral capsids were both injected retro-orbitally at 1E12 vg/mouse (in 100 μL volume) with 2 mg/mL of doxycycline given in drinking water every other week